Apologies for the lack of posting.
It's been a busy few months. I have, however, been writing, just not posting stuff to the blog. Some of the writing is for a longer book project.
I did, though, publish a column over at DTC Perspectives giving my take on what I have dubbed "Social Media Guidance Year." This of course comes on the heels of the new national holiday, June 17, which shall henceforward be known as Social Media Guidance Day.
Check it out: http://www.dtcperspectives.com/dtc-news/social-media-guidance-year.html
Master Class Early-Bird Discount Expiring Soon!
There are still spaces available in the November 12 & 18 sessions of Using Social Media Compliantly in Princeton, NJ, and Boston, MA, respectively.
These classes will go beyond the information presented in my recent articles (available here and here) about the FDA guidances, and focus on the key decisions that need to be made on the top three platforms (Facebook, Twitter, and YouTube) to use these platforms compliantly.
Right now, there is a $100 early-bird discount available, but that is about to expire, so if you're interested in attending, register now!
These classes will go beyond the information presented in my recent articles (available here and here) about the FDA guidances, and focus on the key decisions that need to be made on the top three platforms (Facebook, Twitter, and YouTube) to use these platforms compliantly.
Right now, there is a $100 early-bird discount available, but that is about to expire, so if you're interested in attending, register now!
"Reminder-like" Promotions
This week at the Food & Drug Law Institute's Advertising & Promotion Conference, FDA made a subtle, but vital, clarification in the context of discussing the scope of its guidance on space-limited contexts.
The clarification came on the penultimate slide* of FDA's presentation about the space-limited and correcting misinformation guidances. On that slide, and during that portion of the corresponding presentation, FDA noted that the guidance explicitly set aside any discussion of reminder advertising, and addressed the prohibition for use of the reminder ad format by sponsors of black box drugs.
FDA then proceeded to note that although reminder advertising and promotion is prohibited for black box drugs, the FDA has long recognized in traditional (i.e., offline) communications the ability of sponsors of black box drugs to engage in "reminder-like" promotion.
The key distinction for reminder-like promotion vs. reminder promotion is that reminder-like promotion must have an"[a]ccompanying PI or brief summary" and a statement to "Please see..." the accompanying PI. Reminder promotions do not have such requirements.
At this point, it's useful to briefly recap the traditional reminder advertising/promotion requirements:
As I discussed in my article on search engine marketing, traditional reminder advertising has only a few required elements:
- brand name (if any)
- generic name of the product and/or active ingredients
All other elements are optional. Quoting directly from my article, the optional elements are:
- quantitative ingredient statements (e.g., 20 mg)
- dosage form (e.g., tablets or capsules)
- quantity of package contents (e.g., 30 pills per bottle)
- price
- name and address of the manufacturer, packer or distributor
- other information so long as it makes “no representation or suggestion” about the product use
So, FDA is acknowledging this week the existence of a separate category of communication with a distinct set of requirements. For reminder-like promotion, the required elements appear to be:
- Brand name (if any)
- Generic name of the product and/or active ingredients
- "Please see..." statement directing people to the PI or Brief Summary
- Provision of a PI or Brief Summary immediately accompanying the advertisement
This presentation was a welcome clarification for many reasons. Perhaps the most important reason is that many people in industry have been concerned that FDA's guidance provisions make it impossible for sponsors of black box products to engage in communication channels with space limitations.
Second, many of the communications that seem most appropriate in social media, and other space-limited contexts would most likely make use of formats that fall under the category of reminder or "reminder-like" communications, such as sending a Tweet to a customer who is asking where she/he can find information about the most common side effects associated with a drug.
There are, though, still many questions to be answered about these "reminder-like" communications. Among those that leap immediately to mind are:
- What are the rules about what you may or may not include in a reminder-like promotion?
- Are you permitted to include all of the same information in a reminder-like promotion as in a traditional reminder ad for a drug without a black box?
- Are there any additional requirements for the reminder-like ads beyond the link or physical accompaniment of the prescribing information?
- When engaging in reminder-like promotion online, must the link to the PI be a direct link, or would it suffice to have a link to page where a link to the full PI is presented along with additional information?
- Is it permitted (or required) to mention that the product has black box warnings in the reminder-like promotion?
- If either, is there specific language that should be used to describe those warnings?
These last few questions are particularly important for the context of space-limited contexts explicitly addressed by the guidance because the current Google Black Box ad format makes use of the following phrase, which sponsors are not permitted to alter:
"Click to see full safety and prescribing information, including boxed warning. More info"
Many people (including me) think that this language is confusing in consumer-directed promotion because consumers probably aren't familiar with either a PI or the phrase "boxed warning."
So, although that language is appropriate for HCP advertising, it seems as if a more consumer-friendly version should exist.
For example, "Click to see full product benefit and risk information, including all serious warnings. More info"
I hope this recent presentation by FDA furthers the conversation about how sponsors of black box products can make use of space-limited contexts while remaining compliant with FDA promotional regulations.
* Note that I'm not sure whether the slides will be available for non-FDLI members and non-conference attendees. There does not appear to be any log-in requirement to access this link. FDA generally makes their public presentations available, so if the link is blocked for any users, contact me and I'll let you know when FDA posts the presentations.
Podcast with PharmaGuy
I was interviewed for PharmaGuy's podcast today. One of the primary topics was the FDAnews Ad-promo workshop that I'm teaching October 15-16. It was a fun time, and here's the archive.
Check Out Business Podcasts at Blog Talk Radio with Pharmaguy on BlogTalkRadio
New Speaking Engagements
I have accepted an invitation to speak at the DTC Point of Care Conference on October 1st in Baltimore. Mukesh Mehta of PDR and I will be talking about the explosive growth in EHRs and the need to engage this new communication channel compliantly for providing patients the information they need while ensuring compliance.
Also, I have partnered with FDAnews to prepare a two-day workshop for ad/promo professionals. This workshop will present the basic principles that apply to all promotional communications. In addition, I'll be focusing on some of the areas that have been subject to recent clarification and guidance, including social media and product name usage.
FDAnews is offering a discount exclusively to readers of this blog. To get the details, fill out the contact form in the right rail and ask me for the discount code.
Also, I have partnered with FDAnews to prepare a two-day workshop for ad/promo professionals. This workshop will present the basic principles that apply to all promotional communications. In addition, I'll be focusing on some of the areas that have been subject to recent clarification and guidance, including social media and product name usage.
FDAnews is offering a discount exclusively to readers of this blog. To get the details, fill out the contact form in the right rail and ask me for the discount code.
An Underutilized Phrase in SEM?
I suspect that "FDA-approved," "FDA-cleared," "approved by the FDA," and similar phrases are not fully appreciated by marketers of prescription products.
There is a tendency of marketers of prescription products to put on their blinders and see themselves and their product's performance solely in the context of other prescription products. Who's one slot above us on the IMS Health Rankings and who's one slot below? Are we gaining or losing ground?
But for consumers (and even HCPs for certain conditions), the decision set (your actual competitors in the marketplace) include many non-prescription, and even non-drug treatments. Dietary supplements, homeopathic remedies, and even home cures are legitimate considerations for treating many conditions, and many consumers don't distinguish between all of these categories as rigidly as people in the pharmaceutical industry.
However, the public in general holds the FDA in high esteem, and though criticism of the FDA certainly exists, most consumers consider the fact that the FDA has reviewed and approved (or cleared) a product as a guarantee of the product's quality and overall safety.
As such, the phrase "FDA-approved" can have significant value in a context (such as search engine marketing results), where the competition includes many products that are not reviewed, approved, or cleared by the FDA.
As part of FDAMA's passage in 1997, the prohibition on using this phrase was removed from the FD&C Act.
Since the implementation of FDAMA, FDA has not taken any actions for including the phrase "FDA-approved" or its variations in what otherwise would qualify as a reminder ad, and although FDA has never explicitly endorsed the phrase as being compatible with the reminder ad format, it certainly seems to qualify.
A reminder ad may not include any "representation or suggestion relating to the advertised drug product." 21 CFR 202.1(e)(2)(i) And the phrase "FDA-approved" certainly does not seem to violate that prohibition.
There is a tendency of marketers of prescription products to put on their blinders and see themselves and their product's performance solely in the context of other prescription products. Who's one slot above us on the IMS Health Rankings and who's one slot below? Are we gaining or losing ground?
But for consumers (and even HCPs for certain conditions), the decision set (your actual competitors in the marketplace) include many non-prescription, and even non-drug treatments. Dietary supplements, homeopathic remedies, and even home cures are legitimate considerations for treating many conditions, and many consumers don't distinguish between all of these categories as rigidly as people in the pharmaceutical industry.
However, the public in general holds the FDA in high esteem, and though criticism of the FDA certainly exists, most consumers consider the fact that the FDA has reviewed and approved (or cleared) a product as a guarantee of the product's quality and overall safety.
As such, the phrase "FDA-approved" can have significant value in a context (such as search engine marketing results), where the competition includes many products that are not reviewed, approved, or cleared by the FDA.
As part of FDAMA's passage in 1997, the prohibition on using this phrase was removed from the FD&C Act.
Since the implementation of FDAMA, FDA has not taken any actions for including the phrase "FDA-approved" or its variations in what otherwise would qualify as a reminder ad, and although FDA has never explicitly endorsed the phrase as being compatible with the reminder ad format, it certainly seems to qualify.
A reminder ad may not include any "representation or suggestion relating to the advertised drug product." 21 CFR 202.1(e)(2)(i) And the phrase "FDA-approved" certainly does not seem to violate that prohibition.
Redirecting Ads
Updated slightly to correct grammar and spelling, and to improve clarity.
In my article and presentation about search engine marketing for pharmaceutical products, I talk about a category of communications called Redirecting Ads.
These ads have the following characteristics:
In my article and presentation about search engine marketing for pharmaceutical products, I talk about a category of communications called Redirecting Ads.
These ads have the following characteristics:
- The ads link to a product site
- Do not mention a specific product
- Do not imply a specific product
There were many such ads present in the violative materials posted by the FDA in its 2009 enforcement action about paid search, but none of the ads were cited by the FDA as violative. Given that the FDA went out of its way to take such a massive enforcement action and made clear in subsequent statements that this was intended as a clear statement to industry about what was considered unacceptable in this vital medium, it seems clear to me that FDA's silence on Redirecting Ads was deliberate.
The meaning of the silence is debatable. Did FDA think that such ads were outside its purview (this is my view, BTW)? Did FDA think that such ads were unimportant? Did FDA believe these were not prominent enough to merit inclusion in the enforcement action? Did FDA not want to muddy the waters by including Redirecting Ads? Or was it some combination of these different factors? We don't know, and FDA hasn't said anything.
The reason such ads are important (indeed, I would say vital) to people making use of social media is that it can be difficult to include all of the information that FDA requires in a product promotion in certain space-constrained social media platforms. Redirecting Ads avoid the need for meeting all of those requirements by essentially asking people to go to a separate destination where the sponsor CAN meet those requirements.
There are, however, more questions about how to use such an ad format correctly and compliantly. We know from FDA enforcement actions in the past that simply omitting the brand and generic name from an ad does not mean that the ad is considered not to be a product promotion.* That's why I include the characteristic that Redirecting Ads also do not imply a specific product. How though do you avoid such implications?
One clear means of implying a specific product would be via description. You could, for example, state that the ad was for Pfizer's treatment for erectile dysfunction and without ever mentioning the product name, everyone would know which particular brand you were talking about. In the context of HCP-targeted communication, describing the mechanism of action for a product might be another way to clearly identify the product if only one such product exists.
Additionally, some tag lines or other brand assets have developed a prominence and a life of their own to such an extent that simply using the phrases that have become identified with the product constitutes a mention of the brand name or an implication about which product is being discussed. In this context, it is interesting to note some recent television advertising has been running without having received any FDA enforcement action. This advertising clearly leverages brand assets that are identified with a specific product, but nowhere in the ad is the product name mentioned.
I'm glad there is an ad currently running on TV doing this because regardless of this specific execution, it highlights that Redirecting Ads might be vital to social media, but they are in no way limited to social media. Indeed, this advertising format is frequently used in other product categories, such as teaser campaigns for movies, which do not reveal the exact nature of the product in the teaser communications themselves, but instead send people to another location (whether to visit a website or to call a phone number) to learn more.
One of the downsides to Redirecting Ads is that it unfortunately is also a technique used heavily in some of the less savory areas of online advertising and promotion. Indeed, that's one reason that some publications produce lists each year of the "most dangerous celebrities to search" because people pushing malware and viruses attempt to lure visitors by promising one experience in their search campaigns and then directing to other locations.
Recently, I have faced the question of whether Redirecting Ads are inherently limited because their success brings about the end of their utility.
For example, assume a sponsor of Brand X becomes enormously successful running a Redirecting Ad that reads, "Check out www.conditionXtreatment.com to alleviate your worst pain."
The more people click on the link and see the ad (which by definition explicitly discusses a specific product by name and meets all of the requirements for being a product promotion), the more they begin to associate both the website "www.conditionXtreatment.com" and the phrase "alleviate your worst pain" with Brand X. So, does there come a point where using either of those pieces of content becomes synonymous with using the name Brand X itself, and thus the ad that is intended to avoid mention of the product begins to be viewed as implying a specific product and thus violating the third condition for a Redirecting Ad?
It might. Indeed, there are brands whose taglines and marketing messages are probably better known than the brand name itself, but I suspect this problem would only arise after a Redirecting Ad campaign has been in the market for quite a while and thus marketers could feel confident in investing in such campaigns, though they will have to keep an eye out for when the proverbial Rubicon has been crossed.
* Yes, I do realize how convoluted this sentence construction is, but I also think it's necessary to make the point this way.
* Yes, I do realize how convoluted this sentence construction is, but I also think it's necessary to make the point this way.
Targeting a Competitor's Brand Name with Keywords
Although my article and presentation about search engine marketing included a lot of detail, there were some things that I didn't include.
One question that I frequently hear is about the propriety of targeting a competitive product name. In discussing this topic, I need to provide a disclaimer that I am not an attorney, and no advice from me should ever be construed as legal advice.
As for FDA compliance, I think it is perfectly OK to target a competitive brand with SEM ads depending on the type of ads and the way the targeting is conducted.
So, first a bit of background on keywords. From my recent article:
That doesn't mean anything goes, but it does mean that we don't have to recreate the wheel to determine whether it's appropriate to use certain keywords. We simply apply the same principles from the offline world to the online practice.
So, with regard to disease awareness ads (these are ads that lead to disease awareness websites that do not themselves make any representation about a specific product), it is certainly OK to use the name of any product that is approved to treat the condition in question to serve up such ads.
Of course, if the product is being used off-label to treat the condition, then the advertiser might want to be cautious about the use of that keyword, but note that I would not automatically say that it is inappropriate even then.
For example, if a sponsor is currently investigating a product for an indication where there is not any FDA-approved treatments, and in the absence of any approved treatment, use of Product X has become the only resort for treating Condition Y, then a disease awareness campaign seeking to increase public knowledge about Condition Y might include Product X among its keywords, though probably only in conjunction with other keywords or negative keywords to try to target those people who are searching for information about treatments for Condition Y, rather than the audience of people who are searching for information about the approved indication for Product X.
Regarding redirecting ads, black box ad formats, and reminder ads, it would certainly be appropriate to target a competitor's product if both products have an identical indication or an identical population for at least one indication.
To see why, consider the following thought experiment. Imagine a breakthrough drug that creates an entirely new category of treatment. There is a long history of such medicines. Now, imagine a medical journal dedicating a special issue to the 10th anniversary of the approval of the first medicine in that category.
Would there be anything wrong with placing an ad for one of the newer medicines in that category in such an issue? For me, that answer is clearly no. So long as the newer medicine that is being advertised in that issue has the same indication (or a significantly overlapping indication) as the medicine that is the subject of the issue, then it would be OK to place the ad. The ad itself would have to be compliant, regardless of where it was placed, and that would mean that if the ad mentioned the competitive product that was the subject of the issue, then the claims would be comparative and in need of appropriate substantiation, but there's nothing inherently comparative in the placement itself.
The most common objection to such targeting is that such targeting can only be done if there is a head-to-head trial comparing the two drugs. I believe, however, that this conflates the content of the ad with the ad's placement. An ad whose content is not otherwise comparative in nature does not become comparative by virtue of placing it against a competitor.
In the case of a reminder ad or a black box ad, the compliant usage of such an ad format guarantees that there's nothing comparative in its content.
But what about the content of redirecting ads? Well, that's another blog post, and I'll address it tomorrow.
What constraints would I apply to targeting reminder ads or black box ad formats at a competitive product?
I find Venn diagrams the easiest way to explain the different scenarios:
In Scenario 1, Product A & B have identical indications. As I mentioned above, I think it's perfectly acceptable for either product to include its competitor among its keywords and deliver a branded advertising message via a reminder ad or black box ad to the users.
In Scenario 2, everyone who is indicated for Product B is also within the indicated population for Product A. This can happen, for example, when Product A has an indication covering adolescent, adult, and geriatric populations for a condition, but Product B only has an adult indication. My view is that Product A can use Product B as a keyword to deliver branded messaging because by hypothesis everyone indicated for Product B is also indicated for Product A. I do not, however, think the reverse is true. Product B should take precautions such as using "geriatric" and/or "elderly" as negative keywords if Product B wants to include Product A as a keyword.
In Scenario 3, both Product A & Product B might be able to use their competitor as a keyword, but the questions I would want to consider would be things such as how much overlap is there between the two indications, is it possible to distinguish people searching for Product A who ARE indicated for Product B from people searching for Product A who are NOT indicated for Product B. In some cases, such as when products have multiple indications, these questions might be very easily answered. In other cases, it might not be nearly as easy to target the appropriate population.
In all of these cases, my concern is not with the content of the ads themselves. Instead, my concern is that intentionally targeting a population that is not indicated for using my product on label could be evidence of an intent to market the product off-label. So, before engaging in such competitor targeting, I would want to be certain that I could justify my targeting practices as indeed being limited to the currently approved population.
One question that I frequently hear is about the propriety of targeting a competitive product name. In discussing this topic, I need to provide a disclaimer that I am not an attorney, and no advice from me should ever be construed as legal advice.
As for FDA compliance, I think it is perfectly OK to target a competitive brand with SEM ads depending on the type of ads and the way the targeting is conducted.
So, first a bit of background on keywords. From my recent article:
Keywords: Words entered into a search engine by a user. An advertiser identifies keywords that will trigger the appearance of their ad and places a bid for their preferred keywords.
So, keywords and their negative keyword counterparts function analogously to a media plan in traditional media, but instead of buying publications that are read by 18-25 year-old women, an advertiser places an ad in front of people who are entering certain words into their search engine. As such, the same rules that apply to appropriate offline ad placement apply to what makes for an appropriate keyword.Negative Keywords: Words selected by an advertiser that will prevent their ad from appearing.
That doesn't mean anything goes, but it does mean that we don't have to recreate the wheel to determine whether it's appropriate to use certain keywords. We simply apply the same principles from the offline world to the online practice.
So, with regard to disease awareness ads (these are ads that lead to disease awareness websites that do not themselves make any representation about a specific product), it is certainly OK to use the name of any product that is approved to treat the condition in question to serve up such ads.
Of course, if the product is being used off-label to treat the condition, then the advertiser might want to be cautious about the use of that keyword, but note that I would not automatically say that it is inappropriate even then.
For example, if a sponsor is currently investigating a product for an indication where there is not any FDA-approved treatments, and in the absence of any approved treatment, use of Product X has become the only resort for treating Condition Y, then a disease awareness campaign seeking to increase public knowledge about Condition Y might include Product X among its keywords, though probably only in conjunction with other keywords or negative keywords to try to target those people who are searching for information about treatments for Condition Y, rather than the audience of people who are searching for information about the approved indication for Product X.
Regarding redirecting ads, black box ad formats, and reminder ads, it would certainly be appropriate to target a competitor's product if both products have an identical indication or an identical population for at least one indication.
To see why, consider the following thought experiment. Imagine a breakthrough drug that creates an entirely new category of treatment. There is a long history of such medicines. Now, imagine a medical journal dedicating a special issue to the 10th anniversary of the approval of the first medicine in that category.
Would there be anything wrong with placing an ad for one of the newer medicines in that category in such an issue? For me, that answer is clearly no. So long as the newer medicine that is being advertised in that issue has the same indication (or a significantly overlapping indication) as the medicine that is the subject of the issue, then it would be OK to place the ad. The ad itself would have to be compliant, regardless of where it was placed, and that would mean that if the ad mentioned the competitive product that was the subject of the issue, then the claims would be comparative and in need of appropriate substantiation, but there's nothing inherently comparative in the placement itself.
The most common objection to such targeting is that such targeting can only be done if there is a head-to-head trial comparing the two drugs. I believe, however, that this conflates the content of the ad with the ad's placement. An ad whose content is not otherwise comparative in nature does not become comparative by virtue of placing it against a competitor.
In the case of a reminder ad or a black box ad, the compliant usage of such an ad format guarantees that there's nothing comparative in its content.
But what about the content of redirecting ads? Well, that's another blog post, and I'll address it tomorrow.
What constraints would I apply to targeting reminder ads or black box ad formats at a competitive product?
I find Venn diagrams the easiest way to explain the different scenarios:
 |
| Scenario 1: Product A & B have identical indications |
 |
| Scenario 2: Product B population is subset of Product A |
 |
| Scenario 3: Product A & B have overlapping, non-identical indications |
In all of these cases, my concern is not with the content of the ads themselves. Instead, my concern is that intentionally targeting a population that is not indicated for using my product on label could be evidence of an intent to market the product off-label. So, before engaging in such competitor targeting, I would want to be certain that I could justify my targeting practices as indeed being limited to the currently approved population.
Developing Compliant Search Engine Marketing
The week of Google text ads is here, at least for me and readers of this blog.
Last week, I spoke at a CBI social media summit about this topic, and on Monday, my article about the same topic was published by RAPS in its September issue of Regulatory Focus. By the way, this issue was guest edited by John Driscoll, and RAPS members can read more exclusive content at the RAPS website.
As always, I'm posting the article to my Scribd.com page and the presentation to my SlideShare page (links always available in the right rail).
But wait there's more!
All week, I'll be posting on some of the topics that didn't make the final cut for either the article or the presentation because it is definitely worth examining what I regard as the most important promotional tactic in the contemporary marketer's mix.
But wait there's more!
All week, I'll be posting on some of the topics that didn't make the final cut for either the article or the presentation because it is definitely worth examining what I regard as the most important promotional tactic in the contemporary marketer's mix.
By the way, my distribution list received an email about this yesterday. To make sure you don't miss any new developments, just complete the contact form (also in the right rail).
I also offer discounts to speaking engagements and training sessions exclusively to people on my distribution list, and I promise not to spam you. So, hey, sign up for the list.
Finally, keep in mind that I'm available for a training session to help your company understand how to make use of this incredibly important technique and to design policies and procedures to ensure all of your promotional communications are compliant. Just fill out the contact form in the right rail and request a free initial consultation.
Avoiding Cherry Picking
I spoke at CBI's Social Media summit on Friday. It was a good session with lively discussion.
Among the questions that came up was addressing the FDA guidance on correcting misinformation. Attendees were interested in how to avoid the accusation of cherry picking what is correcting.
I emphasized in my response the need to have policies, procedures and documentation to handle this effectively. Specifically, your procedures for correcting misinformation should require (among other things) that the people who are responsible for correcting misinformation must clearly indicate prior to the review being conducted, exactly what they will be reviewing. They might specify a blog post, or a series of comments, or all blog posts between certain dates, etc.
Then, the people should identify ALL misinformation identified within that pre-specified area.
Separately, there needs to be a standard evaluated for determining what level and types of misinformation will be corrected. For example, only correcting misinformation about currently approved indications or correcting all misinformation regarding the product safety and risks but nothing regarding its efficacy.
Finally, the procedure for correcting the misinformation has to be spelled out.
Falling all of these procedures, and applying those procedures and standards to everything that is found without regard for whether the information being corrected is positive or negative about the product should protect companies from the accusation of cherry picking.
Among the questions that came up was addressing the FDA guidance on correcting misinformation. Attendees were interested in how to avoid the accusation of cherry picking what is correcting.
I emphasized in my response the need to have policies, procedures and documentation to handle this effectively. Specifically, your procedures for correcting misinformation should require (among other things) that the people who are responsible for correcting misinformation must clearly indicate prior to the review being conducted, exactly what they will be reviewing. They might specify a blog post, or a series of comments, or all blog posts between certain dates, etc.
Then, the people should identify ALL misinformation identified within that pre-specified area.
Separately, there needs to be a standard evaluated for determining what level and types of misinformation will be corrected. For example, only correcting misinformation about currently approved indications or correcting all misinformation regarding the product safety and risks but nothing regarding its efficacy.
Finally, the procedure for correcting the misinformation has to be spelled out.
Falling all of these procedures, and applying those procedures and standards to everything that is found without regard for whether the information being corrected is positive or negative about the product should protect companies from the accusation of cherry picking.
Why URL Shorteners Matter
Tom commented on a previous post about inVentiv Health's new URL shortener that all of this discussion is moot because we're all being forced to adopt Twitter's shortener anyway, and Marco followed up with some additional clarifications.
Both cite Twitter's support pages about this topic here and here.
So, there's an important correction to the previous post, and there's also a question about why I obsess on character counts and shorteners in general.
First, the correction.
I thought Twitter's 22-character limit on a URL was a maximum, not a minimum. It turns out it's both. No matter how short (or long) a URL is, when it is included in a Tweet, Twitter allocates 22 characters for the link and uses its t.co shortener service to send the user on his/her way to the destination URL.
Importantly, that limitation is different from what displays. A message that is too long will not necessarily display in its entirety, but the URL will count toward only 22 characters of your 140 character total.
Consequently, no URL shortener can offer character savings, and my claim that inVentiv Health was offering a real (though small) savings in the character count was wrong.
However, URL shorteners still matter.
To understand why, you have to first recognize that URL shorteners are just a special case of using URL redirects. URL redirects are simply ways having a user end up a different URL destination than what they click on (or enter into their browser's address bar).
There are many reasons why people make use of redirects. First, websites are constantly evolving and changing. When such changes happen, there's a need to send people using old URLs someplace, and redirects are an option instead of setting up error pages or making people find their way manually to their destination.
Second, some destination URLs are unwieldy. People can find long URLs difficult to read, comprehend, and type. By contrast, a redirect can be much shorter and easier to use.
Third, (and this one matters most to pharmaceutical marketers) there need not be any connection between the information or words provided in the redirecting URL and the eventual destination URL. That matters to pharmaceutical marketers because going back many years, FDA has made clear that usage of a product name in a URL counts as a mention of the product name. And that matters to pharmaceutical marketers because use of a brand name automatically brings with it certain requirements, such as the inclusion of the generic name, whereas a URL redirect enables you to avoid that product mention.
URL shorteners are just one special case of these redirects that provide the benefits of being easier to type. Because URL shorteners work by having an extremely brief root URL while appending a random string of characters to the end, they are not typically easier to read or comprehend.
There has been only one enforcement action from FDA's OPDP for the use of a URL redirect that I am aware of.
FDA made clear that the issue with the ad subject to the enforcement was that the other parts of the ad so clearly identified the product that the mere omission of the product name was not itself sufficient to claim that the ad was not a product promotion. So, one issue when using a redirect (whether shortening or not) is that you cannot simply assume that because the brand name has been removed from the URL that you have thereby prevented your ad from being a product promotion.
If, for example, your company makes only one product in a specific therapeutic category and is well known for doing so, then putting together a message that mentions your company name and the category is likely to be problematic.
Bringing all of this back to inVentiv Health's new shortener, using such a service will not save you characters on Twitter (though of course each platform is unique, and it might offer such a savings on other platforms).
It does, however, provide the advantage of avoiding the mention of a product in the URL that a user sees while still clearly communicating to the user that he or she is going to a webpage for a prescription product that communicates risk information. And these benefits will, to my mind, be more significant if the service becomes standard throughout the industry instead of each company developing their own shortener.
Both cite Twitter's support pages about this topic here and here.
So, there's an important correction to the previous post, and there's also a question about why I obsess on character counts and shorteners in general.
First, the correction.
I thought Twitter's 22-character limit on a URL was a maximum, not a minimum. It turns out it's both. No matter how short (or long) a URL is, when it is included in a Tweet, Twitter allocates 22 characters for the link and uses its t.co shortener service to send the user on his/her way to the destination URL.
Importantly, that limitation is different from what displays. A message that is too long will not necessarily display in its entirety, but the URL will count toward only 22 characters of your 140 character total.
Consequently, no URL shortener can offer character savings, and my claim that inVentiv Health was offering a real (though small) savings in the character count was wrong.
However, URL shorteners still matter.
To understand why, you have to first recognize that URL shorteners are just a special case of using URL redirects. URL redirects are simply ways having a user end up a different URL destination than what they click on (or enter into their browser's address bar).
There are many reasons why people make use of redirects. First, websites are constantly evolving and changing. When such changes happen, there's a need to send people using old URLs someplace, and redirects are an option instead of setting up error pages or making people find their way manually to their destination.
Second, some destination URLs are unwieldy. People can find long URLs difficult to read, comprehend, and type. By contrast, a redirect can be much shorter and easier to use.
Third, (and this one matters most to pharmaceutical marketers) there need not be any connection between the information or words provided in the redirecting URL and the eventual destination URL. That matters to pharmaceutical marketers because going back many years, FDA has made clear that usage of a product name in a URL counts as a mention of the product name. And that matters to pharmaceutical marketers because use of a brand name automatically brings with it certain requirements, such as the inclusion of the generic name, whereas a URL redirect enables you to avoid that product mention.
URL shorteners are just one special case of these redirects that provide the benefits of being easier to type. Because URL shorteners work by having an extremely brief root URL while appending a random string of characters to the end, they are not typically easier to read or comprehend.
There has been only one enforcement action from FDA's OPDP for the use of a URL redirect that I am aware of.
FDA made clear that the issue with the ad subject to the enforcement was that the other parts of the ad so clearly identified the product that the mere omission of the product name was not itself sufficient to claim that the ad was not a product promotion. So, one issue when using a redirect (whether shortening or not) is that you cannot simply assume that because the brand name has been removed from the URL that you have thereby prevented your ad from being a product promotion.
If, for example, your company makes only one product in a specific therapeutic category and is well known for doing so, then putting together a message that mentions your company name and the category is likely to be problematic.
Bringing all of this back to inVentiv Health's new shortener, using such a service will not save you characters on Twitter (though of course each platform is unique, and it might offer such a savings on other platforms).
It does, however, provide the advantage of avoiding the mention of a product in the URL that a user sees while still clearly communicating to the user that he or she is going to a webpage for a prescription product that communicates risk information. And these benefits will, to my mind, be more significant if the service becomes standard throughout the industry instead of each company developing their own shortener.
New Link Shortener for Risk Info
Update: Comments on this post pointed out an error. A new blog post explains that error and provides further information on the use of URL shorteners. http://regulatoryrx.blogspot.com/2014/09/why-url-shorteners-matter.html
inVentiv Health has a new link shortener that appears to make a very valuable contribution to the use of Twitter by pharmaceutical companies.
As I've talked about extensively (e.g., here, here, and here), there are difficulties with trying to follow the FDA social media guidance on the presentation of risk information in space-constrained contexts because of how much stuff FDA wants included in any single message.
Specifically, FDA says messages must include:
1. Brand name
2. Generic name
3. Non-misleading indication statement
4. Abbreviated risk statement
5. Link to complete risk information
In FDA's own example, just including all of the required elements takes up 134 of the 140 characters available for a single message.
inVentiv's solution is quite clever. It provides the benefits of a URL shortener while addressing the objection from FDA that such shorteners tend to obscure the information in the destination.
The shortener from inVentiv appends to a root of "RxRi.sk/" a short character string to a specific page dedicated to risk information. By including both the standard abbreviation of Rx for a prescription drug and taking advantage of the top-level domain for Slovakia (.sk), combined with the two characters Ri, the shortener clearly communicates that the destination location will include risk information about a prescription product without using nearly as many characters as the FDA's example.
In a few tests, the shortened string appears to have a consistent 13 characters vs. the 20 characters in FDA's product name. Of course, the FDA's example included the unnecessary characters "www." at the beginning of the URL, so even FDA's example is really only 16 characters in length.
Is a three-character saving really that big a deal?
Yes, I think it is, and I think there are a few reasons for that. First, every character matters when we're dealing with Twitter. Shaving a few characters here and a few there will add up and make for a far more flexible framework.
Second, inVentiv is making this shortener available to everyone free of charge. Consequently, this can become an industry standard if people are willing to adopt it, and having consistency in such communication platforms will make it far easier for people, especially consumers who don't spend all day thinking about how the FDA regulates prescription drugs, but who just know that Tweets from the medicine they're taking include scary information. For them, gaining familiarity that drugs have risks, and here is where they can find the risks associated with their particular medicine is valuable.
Third, the three-character improvement is for FDA's fictional product name of "NoFocus." FDA's actual recommendation is to always include both the product name and the word "risk" in the URL that directs users to the full risk information. "NoFocus" has seven characters. That seems to be the norm for top brands, as a quick scan of the top 10 selling drugs in the past year reveals three names with six characters, three with seven, and two with eight characters. There are, however, some drugs with much longer names, and if you're marketing a product with 10 or 11 characters in the name (of an extended release version with an "XR" added to the URL) the savings can add up.
Having a standard means of providing this information takes one item off the table when drug names are being created and evaluated.
Of course, some of these benefits are only realized if in fact people adopt inVentiv's shortener and make it a standard. We'll see whether that happens or whether some additional competing services emerge. At the very least, inVentiv has moved the conversation one step forward.
inVentiv Health has a new link shortener that appears to make a very valuable contribution to the use of Twitter by pharmaceutical companies.
As I've talked about extensively (e.g., here, here, and here), there are difficulties with trying to follow the FDA social media guidance on the presentation of risk information in space-constrained contexts because of how much stuff FDA wants included in any single message.
Specifically, FDA says messages must include:
1. Brand name
2. Generic name
3. Non-misleading indication statement
4. Abbreviated risk statement
5. Link to complete risk information
In FDA's own example, just including all of the required elements takes up 134 of the 140 characters available for a single message.
NoFocus (rememberine HCl) for mild to moderate memory loss-May cause seizures in patients with a seizure disorder www.nofocus.com/risk (page 14)And FDA explicitly discourages the use of URL shorteners out of concern that the shorteners will obscure the nature of the information being linked to.
That puts sponsors in a bind because using the FDA's example, there were only six characters for the actual message itself after meeting the regulatory requirements.The Agency does not intend to object to the use of such URL shortening services; however, when possible, the Agency recommends that the URL or web address itself denote to the user that the landing page consists of risk information (e.g., www.product.com/risk). (page 10)
inVentiv's solution is quite clever. It provides the benefits of a URL shortener while addressing the objection from FDA that such shorteners tend to obscure the information in the destination.
The shortener from inVentiv appends to a root of "RxRi.sk/" a short character string to a specific page dedicated to risk information. By including both the standard abbreviation of Rx for a prescription drug and taking advantage of the top-level domain for Slovakia (.sk), combined with the two characters Ri, the shortener clearly communicates that the destination location will include risk information about a prescription product without using nearly as many characters as the FDA's example.
In a few tests, the shortened string appears to have a consistent 13 characters vs. the 20 characters in FDA's product name. Of course, the FDA's example included the unnecessary characters "www." at the beginning of the URL, so even FDA's example is really only 16 characters in length.
Is a three-character saving really that big a deal?
Yes, I think it is, and I think there are a few reasons for that. First, every character matters when we're dealing with Twitter. Shaving a few characters here and a few there will add up and make for a far more flexible framework.
Second, inVentiv is making this shortener available to everyone free of charge. Consequently, this can become an industry standard if people are willing to adopt it, and having consistency in such communication platforms will make it far easier for people, especially consumers who don't spend all day thinking about how the FDA regulates prescription drugs, but who just know that Tweets from the medicine they're taking include scary information. For them, gaining familiarity that drugs have risks, and here is where they can find the risks associated with their particular medicine is valuable.
Third, the three-character improvement is for FDA's fictional product name of "NoFocus." FDA's actual recommendation is to always include both the product name and the word "risk" in the URL that directs users to the full risk information. "NoFocus" has seven characters. That seems to be the norm for top brands, as a quick scan of the top 10 selling drugs in the past year reveals three names with six characters, three with seven, and two with eight characters. There are, however, some drugs with much longer names, and if you're marketing a product with 10 or 11 characters in the name (of an extended release version with an "XR" added to the URL) the savings can add up.
Having a standard means of providing this information takes one item off the table when drug names are being created and evaluated.
Of course, some of these benefits are only realized if in fact people adopt inVentiv's shortener and make it a standard. We'll see whether that happens or whether some additional competing services emerge. At the very least, inVentiv has moved the conversation one step forward.
Get on the list!
September is always a busy month. I'll be publishing two articles and speaking at three conferences. As materials come available, I'll post them to my Scribd.com or SlideShare pages.
The links to these pages are in the right rail, and you can bookmark them.
I also maintain a distribution list of people who would like to receive updates from me. If you want to save yourself some time, then just complete the Contact Form on the right, and I'll add you to my distribution list. As new materials become available, or as important news warrants, I will send out updates directly to your inbox.
I promise these updates won't flood your inbox, and you can subscribe at any time.
One additional benefit of being on the distribution list (aside from being the first to know that there is new information) is that I will be making special discounts to my speaking engagements and training sessions available only to people on my distribution list and PhillyCooke Consulting clients.
So, to qualify for these discounts, fill out that Contact Form and request that you be added to the list.
And, of course, if you happen to need any of these services, please mention that on your Contact Form, and I'll set up time for your free initial consultation.
The links to these pages are in the right rail, and you can bookmark them.
I also maintain a distribution list of people who would like to receive updates from me. If you want to save yourself some time, then just complete the Contact Form on the right, and I'll add you to my distribution list. As new materials become available, or as important news warrants, I will send out updates directly to your inbox.
I promise these updates won't flood your inbox, and you can subscribe at any time.
One additional benefit of being on the distribution list (aside from being the first to know that there is new information) is that I will be making special discounts to my speaking engagements and training sessions available only to people on my distribution list and PhillyCooke Consulting clients.
So, to qualify for these discounts, fill out that Contact Form and request that you be added to the list.
And, of course, if you happen to need any of these services, please mention that on your Contact Form, and I'll set up time for your free initial consultation.
Google Text Ads Revisited
I just submitted a draft of my article about Google text ads for the September issue of RAPS Regulatory Focus. I was surprised at how much has changed since my initial piece on this topic about two years ago.
If RAPS agrees, I'll make a copy available on my Scribd.com page (which is always accessible via the link in the right rail of the blog).
I'll also be drawing upon much of that material for a presentation I'm delivering on September 12 at the CBI Social Media Summit in Philadelphia. I hope you'll join us.
If RAPS agrees, I'll make a copy available on my Scribd.com page (which is always accessible via the link in the right rail of the blog).
I'll also be drawing upon much of that material for a presentation I'm delivering on September 12 at the CBI Social Media Summit in Philadelphia. I hope you'll join us.
Eliminating the Disparity in 2253 Filing Requirements
One of the difficulties with the guidance on Fulfilling Regulatory Requirements for Postmarketing Submissions of Interactive Promotional Media for Prescription Human and Animal Drugs and Biologics (or the 2253 guidance as I call it*) on social media is that it establishes a distinction in filing requirements between forums that are publicly accessible and those that are not.
Sponsors of prescription products who wish to engage in social media are granted two pathways via this guidance for meeting their 2253 filing requirements, which is the obligation to submit all promotional materials at the time of first use or prior to initial dissemination.
For forums that are publicly accessible, sponsors can submit the initial communication on a third-party site, along with any home or profile page, and the URL where the real-time communications will occur. Then, every month, firms should submit the list of all URLs where it is engaging in real-time communications.
However, forums that are not publicly accessible have a different requirement. Instead of merely submitting a list of URLs each month, FDA requests that sponsors who choose to use such forums submit screenshots of the interactions that occur.
It has been pointed out by others (including yours truly) that this distinction is difficult to understand or draw in practice. For example, many platforms have a minimal requirement that to view any discussions in the platform, you must be a member of (and logged in to your account on) the platform. Would such a requirement be seen by the FDA as making the site not publicly accessible?
Moreover, for third-party sites, this requirement might change without notice. Were that to happen, a sponsor could go from being in compliance to being out of compliance without having themselves made any changes, and that seems odd. It certainly seems that what a sponsor is operating compliantly should not depend on the policies of a third-party site.
Another difficulty is that the disparity in filing requirement is huge and would put a barrier in front of participating in non-restricted forums. Preparing all of the screenshots on a monthly basis could be a significant undertaking depending on the volume of the communications in the forum. While that might seem desirable at first blush, there are good reasons to want to operate such closed forum. Such forums enable, for example, full verification of credentials prior to admission. That means that information can be more freely shared without concern that people who should not have access to the information might receive it. For example, if I were the sponsor of a pain medication, I might want to discuss methods that some patients are using to circumvent abuse prevention mechanisms, but obviously I would not want that information to be available in a publicly accessible forum.
The essence behind this requirement seems to be the desire of FDA to monitor the real-time communications and to rely on the possibility of FDA monitoring of communications to ensure that all communications are appropriate.**
One way of addressing these concerns and eliminating the disparity in filing requirements is to require that when sponsors make use of third-party platforms with restricted access that they set up credentials for FDA to access the forum.
It would be easy for FDA to create an email address and name (e.g., OPDP Social Media & OPDPSocMed@FDA.gov) that could be created as a user for any restricted access platforms. To further simplify things, FDA could establish and publish a list of its credentials on the most prominent forums (e.g., YouTube, Facebook, LinkedIn, Twitter) for monitoring purposes. In that way, sponsors could simply create a user account with FDA's credentials when they set up their restricted-access forums. The sponsor could then include as part of their initial 2253 filing requirement a password (if required) for FDA to access the forum.
From that point forward, sponsors would then be on equal footing with the requirement simply to file monthly reports indicating their continued involvement in the platform but not being required to submit screenshots of all of the interactions.
This method would enable FDA to monitor the conversations in real-time without creating a disparity in filing or a barrier to establishing and maintaining restricted-access forums. It also would ensure that changes made by third-parties could not cause a sponsor to find themselves out of compliance with FDA requirements.
* It is worth noting that the guidance also affects filing of FDA Form 2301 for veterinary medicines. Nothing in this post is affected by this nuance.
** Quoting from the guidance, "if a site has restricted access and, as such, FDA may not have access to the site" (page 7, emphasis added)
Sponsors of prescription products who wish to engage in social media are granted two pathways via this guidance for meeting their 2253 filing requirements, which is the obligation to submit all promotional materials at the time of first use or prior to initial dissemination.
For forums that are publicly accessible, sponsors can submit the initial communication on a third-party site, along with any home or profile page, and the URL where the real-time communications will occur. Then, every month, firms should submit the list of all URLs where it is engaging in real-time communications.
However, forums that are not publicly accessible have a different requirement. Instead of merely submitting a list of URLs each month, FDA requests that sponsors who choose to use such forums submit screenshots of the interactions that occur.
It has been pointed out by others (including yours truly) that this distinction is difficult to understand or draw in practice. For example, many platforms have a minimal requirement that to view any discussions in the platform, you must be a member of (and logged in to your account on) the platform. Would such a requirement be seen by the FDA as making the site not publicly accessible?
Moreover, for third-party sites, this requirement might change without notice. Were that to happen, a sponsor could go from being in compliance to being out of compliance without having themselves made any changes, and that seems odd. It certainly seems that what a sponsor is operating compliantly should not depend on the policies of a third-party site.
Another difficulty is that the disparity in filing requirement is huge and would put a barrier in front of participating in non-restricted forums. Preparing all of the screenshots on a monthly basis could be a significant undertaking depending on the volume of the communications in the forum. While that might seem desirable at first blush, there are good reasons to want to operate such closed forum. Such forums enable, for example, full verification of credentials prior to admission. That means that information can be more freely shared without concern that people who should not have access to the information might receive it. For example, if I were the sponsor of a pain medication, I might want to discuss methods that some patients are using to circumvent abuse prevention mechanisms, but obviously I would not want that information to be available in a publicly accessible forum.
The essence behind this requirement seems to be the desire of FDA to monitor the real-time communications and to rely on the possibility of FDA monitoring of communications to ensure that all communications are appropriate.**
One way of addressing these concerns and eliminating the disparity in filing requirements is to require that when sponsors make use of third-party platforms with restricted access that they set up credentials for FDA to access the forum.
It would be easy for FDA to create an email address and name (e.g., OPDP Social Media & OPDPSocMed@FDA.gov) that could be created as a user for any restricted access platforms. To further simplify things, FDA could establish and publish a list of its credentials on the most prominent forums (e.g., YouTube, Facebook, LinkedIn, Twitter) for monitoring purposes. In that way, sponsors could simply create a user account with FDA's credentials when they set up their restricted-access forums. The sponsor could then include as part of their initial 2253 filing requirement a password (if required) for FDA to access the forum.
From that point forward, sponsors would then be on equal footing with the requirement simply to file monthly reports indicating their continued involvement in the platform but not being required to submit screenshots of all of the interactions.
This method would enable FDA to monitor the conversations in real-time without creating a disparity in filing or a barrier to establishing and maintaining restricted-access forums. It also would ensure that changes made by third-parties could not cause a sponsor to find themselves out of compliance with FDA requirements.
* It is worth noting that the guidance also affects filing of FDA Form 2301 for veterinary medicines. Nothing in this post is affected by this nuance.
** Quoting from the guidance, "if a site has restricted access and, as such, FDA may not have access to the site" (page 7, emphasis added)
Prescription Products Don't Exist, But I Talk About Them Anyway
I use the phrase "prescription products" quite frequently. Technically, I'm misusing the phrase, but I prefer to think of it as a shorthand term of art. By "prescription products," I mean all of those products whose promotional labeling and advertising is subject to FDA regulation. That includes prescription drugs, biologics, vaccines, and restricted medical devices.
Of course, vaccines are not usually limited by prescription, and it doesn't make sense to speak about a prescription requirement for many restricted medical devices, but I find the smaller two-word phrase less cumbersome than the rather bulky "all of those products whose promotional labeling and advertising is subject to FDA regulation."
I prefer the slight fiction of creating my own term to the more inaccurate phrase of FDA-regulated products because that broader phrase includes, of course, over-the-counter drugs, homeopathic substances, nutritional supplements, tobacco, and even food and cosmetics; however, these other products are not subject to FDA regulation of their promotional efforts, except in so far as FDA requires that their promotional efforts cannot imply that the items are in fact prescription products.
Of course, vaccines are not usually limited by prescription, and it doesn't make sense to speak about a prescription requirement for many restricted medical devices, but I find the smaller two-word phrase less cumbersome than the rather bulky "all of those products whose promotional labeling and advertising is subject to FDA regulation."
I prefer the slight fiction of creating my own term to the more inaccurate phrase of FDA-regulated products because that broader phrase includes, of course, over-the-counter drugs, homeopathic substances, nutritional supplements, tobacco, and even food and cosmetics; however, these other products are not subject to FDA regulation of their promotional efforts, except in so far as FDA requires that their promotional efforts cannot imply that the items are in fact prescription products.
PhillyCooke.com Is Now Live
As many of you know, I have recently launched an independent consulting practice, focused on helping companies figure out how to use new and emerging digital channels to provide information about FDA-regulated products to the people who need that information, whether that's patients, caregivers, or doctors and other healthcare professionals.
I'm in the process of forming an official S-Corporation (paperwork filed with and apparently accepted by the IRS and Commonwealth of Pennsylvania) and this blog has been undergoing some design changes as I've adapted it to become simply one page on the slightly larger website that is dedicated to the consulting practice.
You might have noticed the change in the page header, new navigation elements, background image, etc.
Aside from the cosmetic changes, the intention is to provide a single location where you can find information about the topic of promoting FDA-regulated products and the services I provide as a consultant. So, there's a new page dedicated to describing the services PhillyCooke Consulting offers.
If you find yourself in need of any of the services, please reach out to me. You can fill out the contact form on the right, and you'll hear from me as soon as I am able to do so.
And if you're not sure whether your specific request falls within the scope of services I provide, then please ask. I don't do everything, but I am a committed provider of referrals and would happily connect you with someone else in my Rolodex.
In addition, this blog will continue, and I hope you will continue to read it. Because although there's a change on my business card, I remain dedicated to the endlessly fascinating topic of FDA regulation of advertising and promotion of prescription products.
I'm in the process of forming an official S-Corporation (paperwork filed with and apparently accepted by the IRS and Commonwealth of Pennsylvania) and this blog has been undergoing some design changes as I've adapted it to become simply one page on the slightly larger website that is dedicated to the consulting practice.
You might have noticed the change in the page header, new navigation elements, background image, etc.
Aside from the cosmetic changes, the intention is to provide a single location where you can find information about the topic of promoting FDA-regulated products and the services I provide as a consultant. So, there's a new page dedicated to describing the services PhillyCooke Consulting offers.
If you find yourself in need of any of the services, please reach out to me. You can fill out the contact form on the right, and you'll hear from me as soon as I am able to do so.
And if you're not sure whether your specific request falls within the scope of services I provide, then please ask. I don't do everything, but I am a committed provider of referrals and would happily connect you with someone else in my Rolodex.
In addition, this blog will continue, and I hope you will continue to read it. Because although there's a change on my business card, I remain dedicated to the endlessly fascinating topic of FDA regulation of advertising and promotion of prescription products.
YouTube Channel Launched
I'm always looking to expand in my use of social media and to provide more resources for others interested in using social media for promoting prescription products, so I just launched PhillyCooke's YouTube Channel.
Here is the link, which will also be featured in the widget on the right with the other online locations where you can find resources from me.
Right now, there is only one video, a short clip from the movie The Social Network, that I really liked because as much as I love social media, I have sometimes found it a frustrating experience, as I've talked about before, trying to figure out how all of this stuff works.
Here is the link, which will also be featured in the widget on the right with the other online locations where you can find resources from me.
Right now, there is only one video, a short clip from the movie The Social Network, that I really liked because as much as I love social media, I have sometimes found it a frustrating experience, as I've talked about before, trying to figure out how all of this stuff works.
Tweet Embedding Correction
My previous post on Twitter's new Tweet embedding feature contained an error that was pointed out to me by a correspondent.
I claimed that one of the drawbacks to the way Twitter was implementing Tweet embedding is that for the feature to function, you must include the full URL of the Tweet you want to embed in the new Tweet. As I wrote:
In fact, Twitter automatically shortens the URL, so that it is not the full character count of the URL that counts against the 140-character limit for each Tweet.
However, the character count for the URL is still not as short as a typical URL shortener.
Here were the results of playing with the functionality:
Again, I used the same Tweet for the embedding:
That Tweet is available at: https://twitter.com/PhillyCooke/status/496670428187090944
The full URL is 57-characters long.
However, I can add it to a Tweet that only appears to have 24 characters remaining (i.e., the rest of the Tweet is actually 116-characters long).
Note that I'm able to comfortably insert the 57-character URL into the Tweet, and the "Tweet" button is still functioning, with an available character count of 0.
Of course, the 23 characters required by Twitter's URL shortening of its own URL is still greater than the 11 characters that I was able to get using Ow.ly, but rather than being a saving of 46 characters, as I claimed in the original post, it is a mere 12 characters that are saved.
Even so, 12 characters does constitute nearly 10% of a Tweet's 140 total characters. Such a savings is not to be ignored lightly.
Thanks to the correspondent who told me about the error, and let me know via email and/or comments if you have any other ideas for saving characters or learning more about how Twitter's embedding functionality works.
I claimed that one of the drawbacks to the way Twitter was implementing Tweet embedding is that for the feature to function, you must include the full URL of the Tweet you want to embed in the new Tweet. As I wrote:
To get the embedding to function as demonstrated above, you must include the full URL of the original Tweet in the new Tweet....the full URL is extremely long. In my example it takes up 57 characters of the 140 allowed...That was incorrect.
In fact, Twitter automatically shortens the URL, so that it is not the full character count of the URL that counts against the 140-character limit for each Tweet.
However, the character count for the URL is still not as short as a typical URL shortener.
Here were the results of playing with the functionality:
Again, I used the same Tweet for the embedding:
 |
| Original Tweet |
That Tweet is available at: https://twitter.com/PhillyCooke/status/496670428187090944
The full URL is 57-characters long.
However, I can add it to a Tweet that only appears to have 24 characters remaining (i.e., the rest of the Tweet is actually 116-characters long).
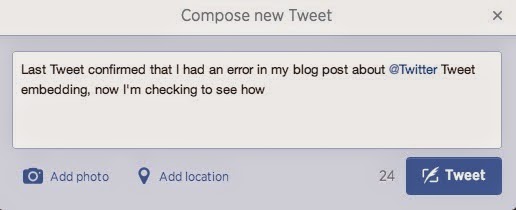 |
| New Tweet Prior to Adding the URL |
Note that I'm able to comfortably insert the 57-character URL into the Tweet, and the "Tweet" button is still functioning, with an available character count of 0.
 |
| Tweet with Full URL |
Also note that the Tweet URL took up 23 characters because I had to include a space between the last word "how" and the URL. Otherwise, Twitter doesn't acknowledge the URL as being an embedded Tweet and simply prevents me from sending out the Tweet.
 |
| Tweet without Space Showing It Is Too Long |
In this case, you can see that the URL isn't recognized as such by Twitter. Consequently, the character count shows up as -33, and the "Tweet" button is grayed out showing that it is inactive.
All of the above screen shots were taken from Twitter.com on a Macbook Air using Chrome.
I also checked how this functionality worked on HootSuite, which I have previously mentioned is my preferred means of accessing Twitter on my laptop. And the same shortening behavior happened.
 |
| Tweet on Hootsuite Prior to Sending |
Of course, the 23 characters required by Twitter's URL shortening of its own URL is still greater than the 11 characters that I was able to get using Ow.ly, but rather than being a saving of 46 characters, as I claimed in the original post, it is a mere 12 characters that are saved.
Even so, 12 characters does constitute nearly 10% of a Tweet's 140 total characters. Such a savings is not to be ignored lightly.
Thanks to the correspondent who told me about the error, and let me know via email and/or comments if you have any other ideas for saving characters or learning more about how Twitter's embedding functionality works.
Social Media Webinar
I'm presenting a webinar for LSTI on Wednesday, October 8.
In the webinar, I'll be taking a step back from the individual guidances we've received and try to give marketers and others interested in the use of social media for promoting prescription products the 50,000-foot view.
By doing so, I hope to provide a roadmap for people who are struggling to determine what they can expect from FDA and how to move forward on implementing social media initiatives.
Here's the link to the full description and registration information: http://www.lifesciencetraininginstitute.com/doc/fda-guidance-on-social-media-questions-answered-and-unanswered-0001
Hope you'll join!
In the webinar, I'll be taking a step back from the individual guidances we've received and try to give marketers and others interested in the use of social media for promoting prescription products the 50,000-foot view.
By doing so, I hope to provide a roadmap for people who are struggling to determine what they can expect from FDA and how to move forward on implementing social media initiatives.
Here's the link to the full description and registration information: http://www.lifesciencetraininginstitute.com/doc/fda-guidance-on-social-media-questions-answered-and-unanswered-0001
Hope you'll join!
PHL RAPS Meeting September 9
Update: Registration is now live: http://www.raps.org/EventDetail.aspx?id=19972#horizontalTab3
Our next local RAPS meeting will be held Tuesday, September 9, again at the Primavera Pizza Kitchen in Ardmore. The event will lead up to the RAPS national conference in Austin, TX, Sept. 27 – Oct. 1. Here’s the online registration for the national conference: http://connect.raps.org/2014raps/home/?ssopc=1
Linda Bowen of sanofi will be joining us to present conference overview, and Alec Gaffney, who reports for RAPS Regulatory Focus, will be providing an exclusive preview of the panel discussion he’s participating in at RAPS national about using technology for regulatory intelligence.
Some of you might know Alec for his recently published lists of 460 to Follow and RSS Feeds for Regulatory Pros. I'm proud to say that I'm included on both lists and am really looking forward to hearing from Alec himself on how he manages to stay on top of so much information.
Our next local RAPS meeting will be held Tuesday, September 9, again at the Primavera Pizza Kitchen in Ardmore. The event will lead up to the RAPS national conference in Austin, TX, Sept. 27 – Oct. 1. Here’s the online registration for the national conference: http://connect.raps.org/2014raps/home/?ssopc=1
Linda Bowen of sanofi will be joining us to present conference overview, and Alec Gaffney, who reports for RAPS Regulatory Focus, will be providing an exclusive preview of the panel discussion he’s participating in at RAPS national about using technology for regulatory intelligence.
Some of you might know Alec for his recently published lists of 460 to Follow and RSS Feeds for Regulatory Pros. I'm proud to say that I'm included on both lists and am really looking forward to hearing from Alec himself on how he manages to stay on top of so much information.
Embedding Tweets?
Update: This post contains an error about the character counts of the URLs. That error was corrected via a more recent post, which can be accessed here: http://regulatoryrx.blogspot.com/2014/08/tweet-embedding-correction.html
Last week, Media Bistro had a story about Twitter enabling the embedding of Tweets, and several people asked me whether this would be useful for pharma to make use of social media and provide more information.
Here is a sample progression that I sent out much to my followers' annoyance (sorry about that!).
Original Tweet:
You won't always see exactly that same thing.
For example, here's what the Tweet looked like in my Hootsuite tab (again from Chrome on Macbook Air):
Again, the full original Tweet is displayed with full attribution (Twitter handle, user name, and Twitter avatar).
Tapping the new Tweet (with the embedded Tweet inside it) takes you to a full screen display of the message.
By contrast, tapping on the embedded Tweet opens only that Tweet in full screen.
From the perspective of achieving compliance for using Twitter for marketing prescription products, I don't see how this will help much. While it is true that some people would be able to receive more information via a Tweet that contains an embedded Tweet (e.g., you could embed a Tweet dedicated solely to providing risk information), the variety in appearance is concerning. I wouldn't feel comfortable relying on the fact that people would access my Tweet via Twitter.com or via the official Twitter app, instead of using a third-party platform, such as Hootsuite.
Indeed, when I'm on my laptop, I almost exclusively use Hootsuite to access Twitter, rather than using Twitter.com; so unless there were a means to restrict access to the message to people using platforms that accommodate Tweet embedding, I'd be averse to relying on this mechanism to provide mandatory information in a Tweet (such as risk information).
I also am not sure whether the FDA would regard information presented via an embedded Tweet as having comparable prominence to information presented in the primary Tweet. That's important for the presentation of risk information to meet the fair balance requirement.
There is another problem with this method of providing information.
To get the embedding to function as demonstrated above, you must include the full URL of the original Tweet in the new Tweet. Here's what that looks like:
As you can see, the full URL is extremely long. In my example it takes up 57 characters of the 140 allowed, and if you use a URL shortener, then the functionality fails. Here's the view on Hootsuite:
Of course, embedded Tweets don't show on Hootsuite, so I also checked on Twitter.com. Here's that view:
And I checked the mobile view in the official Twitter app.
In both cases, only the shortened URL was displayed. That's a real shame as the savings of 46 characters (11 for shortened URL vs. 57 for the full URL) is huge in the realm of Tweets.
When I looked on mobile, I noticed another interesting feature. Namely, that while viewing your own Tweets from the Me portion of the app, the embedded Tweet doesn't appear, even when you provide the full URL. It actually looks the same as the Hootsuite view, presenting just the beginning of the full URL.
So, while embedding Tweets could certainly play a role in providing Twitter users with a way to include much more valuable information in this important platform, I'm afraid that at the moment it won't significantly expand the ability to use Twitter compliantly for manufacturers of prescription products.
h/t to Alec Gaffney & Polaris Consulting for this post.
Last week, Media Bistro had a story about Twitter enabling the embedding of Tweets, and several people asked me whether this would be useful for pharma to make use of social media and provide more information.
Here is a sample progression that I sent out much to my followers' annoyance (sorry about that!).
Original Tweet:
 |
| Original Tweet on Twitter.com |
Here's the Tweet with the embedded portion as it appears on Twitter.com accessed from my Macbook Air using Chrome:
 |
| Embedded Tweet on Twitter.com |
You won't always see exactly that same thing.
For example, here's what the Tweet looked like in my Hootsuite tab (again from Chrome on Macbook Air):
 |
| Embedded Tweet on Hootsuite |
Note the important differences that the embedded Tweet appears fully as a picture-like object with the new Tweet on Twitter.com, but when using Hootsuite, you only see the URL for the original Tweet, and even that URL is cut off, so any information contained in the original Tweet only comes through on some platforms.
The mobile experience is also varied.
I use the official Twitter app on my iPhone.
Here are the different views there. The first is what I see in my Twitter feed from the Home screen, showing my full Twitter stream:
 |
| Mobile View in Stream |
Tapping the new Tweet (with the embedded Tweet inside it) takes you to a full screen display of the message.
 |
| Mobile View 2: Full Screen Display of Tweet |
By contrast, tapping on the embedded Tweet opens only that Tweet in full screen.
 |
| Original Tweet on Mobile from Tapping on Embedded Portion |
From the perspective of achieving compliance for using Twitter for marketing prescription products, I don't see how this will help much. While it is true that some people would be able to receive more information via a Tweet that contains an embedded Tweet (e.g., you could embed a Tweet dedicated solely to providing risk information), the variety in appearance is concerning. I wouldn't feel comfortable relying on the fact that people would access my Tweet via Twitter.com or via the official Twitter app, instead of using a third-party platform, such as Hootsuite.
Indeed, when I'm on my laptop, I almost exclusively use Hootsuite to access Twitter, rather than using Twitter.com; so unless there were a means to restrict access to the message to people using platforms that accommodate Tweet embedding, I'd be averse to relying on this mechanism to provide mandatory information in a Tweet (such as risk information).
I also am not sure whether the FDA would regard information presented via an embedded Tweet as having comparable prominence to information presented in the primary Tweet. That's important for the presentation of risk information to meet the fair balance requirement.
There is another problem with this method of providing information.
To get the embedding to function as demonstrated above, you must include the full URL of the original Tweet in the new Tweet. Here's what that looks like:
 |
| Tweet with Full URL Displayed |
As you can see, the full URL is extremely long. In my example it takes up 57 characters of the 140 allowed, and if you use a URL shortener, then the functionality fails. Here's the view on Hootsuite:
 |
| Embedded Tweet Using URL Shortener on Hootsuite |
Of course, embedded Tweets don't show on Hootsuite, so I also checked on Twitter.com. Here's that view:
 |
| URL Shortener Usage on Twitter.com |
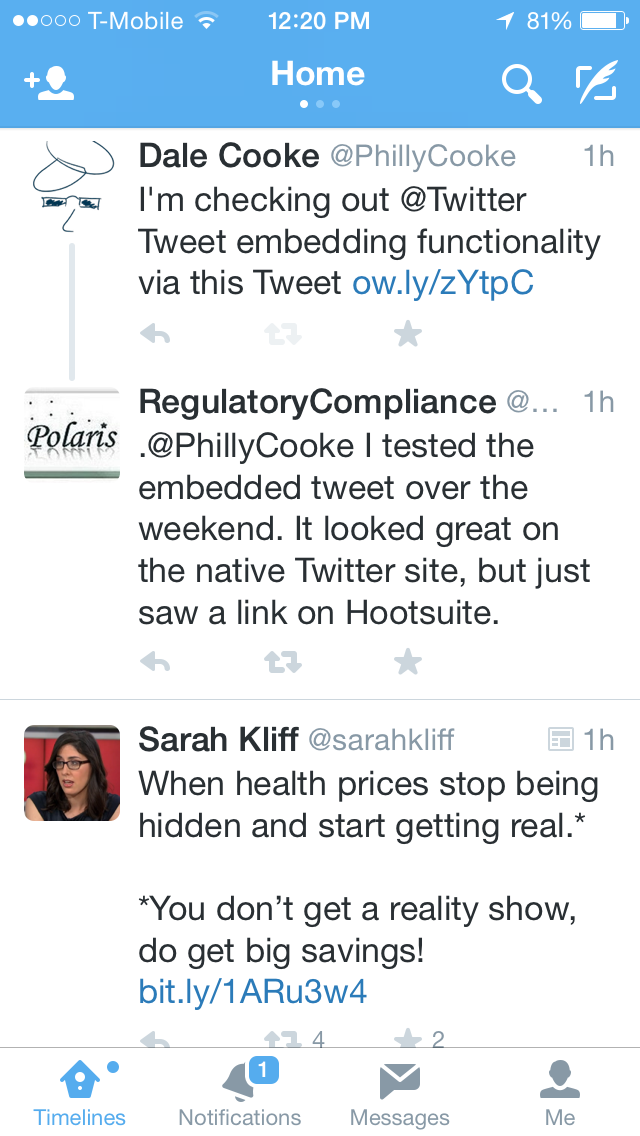 |
| URL Shortener Usage on Mobile in Stream |
In both cases, only the shortened URL was displayed. That's a real shame as the savings of 46 characters (11 for shortened URL vs. 57 for the full URL) is huge in the realm of Tweets.
When I looked on mobile, I noticed another interesting feature. Namely, that while viewing your own Tweets from the Me portion of the app, the embedded Tweet doesn't appear, even when you provide the full URL. It actually looks the same as the Hootsuite view, presenting just the beginning of the full URL.
 |
| Multiple Embedded Tweets on Mobile Showing Shortener Usage |
h/t to Alec Gaffney & Polaris Consulting for this post.
Save Money While Learning about Google Text Ads
As I mentioned previously, I'll be speaking at CBI's one-day event on social media. CBI has confirmed that I can offer a $300 discount to anyone who contacts me via this blog.
Just fill out the contact form on the right, and I'll send you the promotional code by email.
Just fill out the contact form on the right, and I'll send you the promotional code by email.
460 to Follow
Alec Gaffney of RAPS has compiled an extremely valuable list of Twitter accounts for people in regulatory affairs to follow. The list includes governmental accounts, members of the press, industry accounts, and consultants such as yours truly.
I'm very pleased to be included in the list, and if you're looking to start using Twitter for regulatory intelligence, I strongly recommend that you start by combing this list for the accounts that most fit your interests.
Here's the link to the full list: http://www.raps.org/regulatory-focus/twitter/
I'm very pleased to be included in the list, and if you're looking to start using Twitter for regulatory intelligence, I strongly recommend that you start by combing this list for the accounts that most fit your interests.
Here's the link to the full list: http://www.raps.org/regulatory-focus/twitter/
Let's talk about paid search!
For many people, Google serves as the home page of the Internet, so it seems fitting that Google paid search ads and FDA's 2009 enforcement activity started the five-year path to the social media guidances FDA released this year.
I'm currently drafting an article about this topic for the September issue of RAPS Regulatory Focus, which is dedicated to the topic of advertising and promotional regulation, and I just accepted an invitation to speak about the same topic at a conference in Philadelphia on Friday, September 12.
CBI is holding the one-day event, and the agenda is packed with experts and friends, including both industry and consultants. It's certain to be a great event, and I hope you'll join us.
I'm currently drafting an article about this topic for the September issue of RAPS Regulatory Focus, which is dedicated to the topic of advertising and promotional regulation, and I just accepted an invitation to speak about the same topic at a conference in Philadelphia on Friday, September 12.
CBI is holding the one-day event, and the agenda is packed with experts and friends, including both industry and consultants. It's certain to be a great event, and I hope you'll join us.
EyeOnFDA Webinar Available Now
The "Rule" That Isn't
Mark Senak of FleishmanHillard and author of the EyeOnFDA blog presented a webinar on Thursday about the social media guidances. Here's a link to his blog post about it. The presentation itself is not yet available, but during the talk Mark mentioned that he would be posting a recording of the session later. I'll try to let you know when it is available.
During the Q&A of the session, someone asked about the "one click rule."
My experience is that whenever I speak about how FDA applies advertising and promotional regulation to the Internet, someone asks the same question, so I thought it would be useful to have a blog post available that I can simply send people to.
The other thing I find in speaking engagements is that when I poll the audience after I receive the question about the "one click rule" the results are invariably the same. One third of the audience doesn't know what the "one click rule" is, one third is convinced that it's true, and one third knows that the rule is not a rule (hence the scare quotes throughout this post).
So, first, what is this alleged "rule"?
The idea behind the "rule" is that it is OK to present benefit information online so long as risk information is presented within one click (i.e., via a hyperlink) of the benefit information.
Anyone who has been closely following the FDA's discussion of Internet advertising knows that this is false. Indeed, it's safe to say that if someone claims to know anything about FDA regulation of Internet promotion and then endorses the "one click rule," then in fact, that person doesn't know anything about FDA regulation of Internet promotion of prescription products.
Based on John Driscoll's excellent book on FDA ad/promo regulations, I believe the first FDA enforcement action for Internet promotion was in 1998. In that action, the FDA stated that "the link to the full prescribing information alone is insufficient to meet the requirements...that advertisements contain fair balance."
It's been a while since anyone could say that a link could meet the fair balance requirement.
But I can hear at least one person objecting that this isn't fair because the FDA in 1998 was objecting to presenting a link to the PI as a means to meeting the fair balance requirement, but the "one click rule" actually applies to a dedicated link to risk information, not the PI, since the PI contains both benefit AND risk information.
Interestingly, so far as I am aware, the first time FDA discussed having a link to a page dedicated to risk information (and not merely a link to the PI or to the product website more generally) is in the most recent guidance on presenting risk information in space-limited contexts.*
And in that guidance, FDA makes clear that even if a product sponsor includes a link to a page that is dedicated to the presentation of risk information that the link itself will not suffice to meet the fair balance requirement. FDA states that even in the most limited contexts, "[r]isk information should be presented together with benefit information within each individual" communication. In addition, the FDA recommends including a link to a web page that is dedicated to presenting the risk information.
The "one click rule" never did exist, but where did it come from, and why is it one of those zombie ideas that never seems to die?
The origin of such ideas is always difficult to trace. Driscoll cites the FTC disclosure requirements, while Mary Sullivan of Boehringer Ingelheim, presented a talk in 2010 that traced the origin to a comment by FTC on FDA policy.
I don't know that I'm able to add any further light on the subject of its origins, but one important thing to notice about both of these proposed origin stories is that the context is important. In neither case was FDA endorsing the idea. Instead, in the former case, the FTC was adopting its own policy about certain required disclosures, and if there's one word that marketers of prescription products should avoid when discussing the fair balance requirement, it's "disclosure." The fair balance requirement is not a requirement to disclose certain information because that word generally implies that you can meet your obligation by simply making certain information available without regard to its prominence, readability, or likelihood of being perceived and understood by the reader.
In contrast, the fair balance requirement can only be met by making certain that the risks associated with the product are presented with a comparable prominence to the benefit information, and no one could argue that information that is one click away could possibly be as prominent as the information that is in the message itself.
One reason I suspect this idea keeps recurring is that there are other requirements of prescription product promotion that the FDA does think can be met by providing a link. For example, the adequate provision requirement to make the product label with directions for use available is frequently met via the use of links, and in its space-limited guidance, FDA made clear that sponsors can meet the requirements to include a dosage form, quantitative ingredient information via a link to that information rather than providing it in each message.
Perhaps in the pending guidance on the use of hyperlinks, FDA will clarify its rationale for which information must be presented within a message and which information it is permissible to make available via hyperlinks.
Until then (and unfortunately probably long after that), you can just bookmark this blog post and send it to anyone who asks about the "one click rule."
* BTW, if anyone is aware of an enforcement action that does cite a link to a web page dedicated to the risk information, please leave the citation in the comments. Every citation I have been able to find, including of course, the 2009 letters on Google paid search advertising include either links to the PI or just to generic pages on the product website with risk information, but not to a web page dedicated solely to the presentation of risk information.
During the Q&A of the session, someone asked about the "one click rule."
My experience is that whenever I speak about how FDA applies advertising and promotional regulation to the Internet, someone asks the same question, so I thought it would be useful to have a blog post available that I can simply send people to.
The other thing I find in speaking engagements is that when I poll the audience after I receive the question about the "one click rule" the results are invariably the same. One third of the audience doesn't know what the "one click rule" is, one third is convinced that it's true, and one third knows that the rule is not a rule (hence the scare quotes throughout this post).
So, first, what is this alleged "rule"?
The idea behind the "rule" is that it is OK to present benefit information online so long as risk information is presented within one click (i.e., via a hyperlink) of the benefit information.
Anyone who has been closely following the FDA's discussion of Internet advertising knows that this is false. Indeed, it's safe to say that if someone claims to know anything about FDA regulation of Internet promotion and then endorses the "one click rule," then in fact, that person doesn't know anything about FDA regulation of Internet promotion of prescription products.
Based on John Driscoll's excellent book on FDA ad/promo regulations, I believe the first FDA enforcement action for Internet promotion was in 1998. In that action, the FDA stated that "the link to the full prescribing information alone is insufficient to meet the requirements...that advertisements contain fair balance."
It's been a while since anyone could say that a link could meet the fair balance requirement.
But I can hear at least one person objecting that this isn't fair because the FDA in 1998 was objecting to presenting a link to the PI as a means to meeting the fair balance requirement, but the "one click rule" actually applies to a dedicated link to risk information, not the PI, since the PI contains both benefit AND risk information.
Interestingly, so far as I am aware, the first time FDA discussed having a link to a page dedicated to risk information (and not merely a link to the PI or to the product website more generally) is in the most recent guidance on presenting risk information in space-limited contexts.*
And in that guidance, FDA makes clear that even if a product sponsor includes a link to a page that is dedicated to the presentation of risk information that the link itself will not suffice to meet the fair balance requirement. FDA states that even in the most limited contexts, "[r]isk information should be presented together with benefit information within each individual" communication. In addition, the FDA recommends including a link to a web page that is dedicated to presenting the risk information.
The "one click rule" never did exist, but where did it come from, and why is it one of those zombie ideas that never seems to die?
The origin of such ideas is always difficult to trace. Driscoll cites the FTC disclosure requirements, while Mary Sullivan of Boehringer Ingelheim, presented a talk in 2010 that traced the origin to a comment by FTC on FDA policy.
I don't know that I'm able to add any further light on the subject of its origins, but one important thing to notice about both of these proposed origin stories is that the context is important. In neither case was FDA endorsing the idea. Instead, in the former case, the FTC was adopting its own policy about certain required disclosures, and if there's one word that marketers of prescription products should avoid when discussing the fair balance requirement, it's "disclosure." The fair balance requirement is not a requirement to disclose certain information because that word generally implies that you can meet your obligation by simply making certain information available without regard to its prominence, readability, or likelihood of being perceived and understood by the reader.
In contrast, the fair balance requirement can only be met by making certain that the risks associated with the product are presented with a comparable prominence to the benefit information, and no one could argue that information that is one click away could possibly be as prominent as the information that is in the message itself.
One reason I suspect this idea keeps recurring is that there are other requirements of prescription product promotion that the FDA does think can be met by providing a link. For example, the adequate provision requirement to make the product label with directions for use available is frequently met via the use of links, and in its space-limited guidance, FDA made clear that sponsors can meet the requirements to include a dosage form, quantitative ingredient information via a link to that information rather than providing it in each message.
Perhaps in the pending guidance on the use of hyperlinks, FDA will clarify its rationale for which information must be presented within a message and which information it is permissible to make available via hyperlinks.
Until then (and unfortunately probably long after that), you can just bookmark this blog post and send it to anyone who asks about the "one click rule."
* BTW, if anyone is aware of an enforcement action that does cite a link to a web page dedicated to the risk information, please leave the citation in the comments. Every citation I have been able to find, including of course, the 2009 letters on Google paid search advertising include either links to the PI or just to generic pages on the product website with risk information, but not to a web page dedicated solely to the presentation of risk information.
FDA Still Not Ready to Tackle Mobile Promotion
Updated to correct error of publishing an incomplete sentence and a misspelling.
On Thursday, FDA held a webinar about its recent social media guidances. Despite technical difficulties that prevented many (including me) from attending the webinar, FDA did field questions from some of the attendees, and late on Friday afternoon, they posted that Q&A along with the slides from the presentation portion of the event.
In reviewing the Q&A about the space-limited presentation of risk information guidance, one exchange stood out:
While the guidance itself clearly specifies that its scope is limited to social media platforms and paid search with character-count limitations*, it is disappointing to see the exchange. Perhaps it was the nature of the question that resulted in this specific answer, but one would certainly like to know how widely applicable the principles from this guidance are to mobile devices in particular because many of the issues that apply to presentation of risk information in limited character counts also apply to presentation in limited space.
In addition, though, this Q&A further emphasizes one of my concerns about the guidance. It concerns itself solely with the content of each individual Tweet** and completely ignores the surrounding context and nature of Twitter interactions. That larger context is part of what I have begun fleshing out via a previous post about a different Twitter proposal. There is far more going on when a person views a Tweet than just the 140 characters in the Tweet itself, and I believe that FDA is inappropriately limiting both its evaluation of what can make a Tweet compliant (or non-compliant) and its understanding of the actual uses of Twitter and other social media platforms.
Social media hosts public conversations, and just as you would not expect every single sentence uttered to include the complete conversation, you should not necessarily expect every Tweet to include everything needed to achieve a compliant communication.
Further adding to the difficulty is that just as FDA is finally providing guidance to address some of the most pressing issues in social media, we're seeing massive shifts in the consumption of social media itself with an increasing amount of social media interaction happening via mobile devices. So much so that some people have taken to referring to the combined fields as "somo" or "moso" to underline how much the line between social media and mobile has blurred.
* "This draft guidance also does not address responsive web design or other technology-specific layout features that may result in product promotion presentations that differ depending on the technology used to view them (e.g., desktop computer monitors, mobile devices, tablets)." (page 2)
** It's worth noting that people have been referring to this guidance informally as the Twitter guidance, and by refusing to discuss how to apply its principles beyond Twitter and Google, that moniker seems even more appropriate. For years, FDA declaimed its intention to not provide guidance that was limited to any specific platform(s) because of how rapidly the platforms evolved, and yet, ironically, it seems as if FDA is now intentionally doing exactly that.
On Thursday, FDA held a webinar about its recent social media guidances. Despite technical difficulties that prevented many (including me) from attending the webinar, FDA did field questions from some of the attendees, and late on Friday afternoon, they posted that Q&A along with the slides from the presentation portion of the event.
In reviewing the Q&A about the space-limited presentation of risk information guidance, one exchange stood out:
Q1. Does this draft guidance apply to space limitations imposed by mobile devices?A1: This draft guidance does not address technology-specific layout features that may result in product promotion presentations that differ depending on the technology used to view them (e.g., mobile devices, desktop computer monitors, and tablets). The scope of this draft guidance is specific to Internet/social media platforms that impose character space limitations.
While the guidance itself clearly specifies that its scope is limited to social media platforms and paid search with character-count limitations*, it is disappointing to see the exchange. Perhaps it was the nature of the question that resulted in this specific answer, but one would certainly like to know how widely applicable the principles from this guidance are to mobile devices in particular because many of the issues that apply to presentation of risk information in limited character counts also apply to presentation in limited space.
In addition, though, this Q&A further emphasizes one of my concerns about the guidance. It concerns itself solely with the content of each individual Tweet** and completely ignores the surrounding context and nature of Twitter interactions. That larger context is part of what I have begun fleshing out via a previous post about a different Twitter proposal. There is far more going on when a person views a Tweet than just the 140 characters in the Tweet itself, and I believe that FDA is inappropriately limiting both its evaluation of what can make a Tweet compliant (or non-compliant) and its understanding of the actual uses of Twitter and other social media platforms.
Social media hosts public conversations, and just as you would not expect every single sentence uttered to include the complete conversation, you should not necessarily expect every Tweet to include everything needed to achieve a compliant communication.
Further adding to the difficulty is that just as FDA is finally providing guidance to address some of the most pressing issues in social media, we're seeing massive shifts in the consumption of social media itself with an increasing amount of social media interaction happening via mobile devices. So much so that some people have taken to referring to the combined fields as "somo" or "moso" to underline how much the line between social media and mobile has blurred.
* "This draft guidance also does not address responsive web design or other technology-specific layout features that may result in product promotion presentations that differ depending on the technology used to view them (e.g., desktop computer monitors, mobile devices, tablets)." (page 2)
** It's worth noting that people have been referring to this guidance informally as the Twitter guidance, and by refusing to discuss how to apply its principles beyond Twitter and Google, that moniker seems even more appropriate. For years, FDA declaimed its intention to not provide guidance that was limited to any specific platform(s) because of how rapidly the platforms evolved, and yet, ironically, it seems as if FDA is now intentionally doing exactly that.
FDA Webinar Slides & Q&A Now Available
FDA's social media webinar from Thursday had some technical difficulties.
After acknowledging the problems, the FDA then made the slides from the presentation available very rapidly. Late on Friday afternoon, they also posted a transcript of the Q&A.
Here are the links:
Slides
Q&A
After acknowledging the problems, the FDA then made the slides from the presentation available very rapidly. Late on Friday afternoon, they also posted a transcript of the Q&A.
Here are the links:
Slides
Q&A
Subscribe to:
Comments (Atom)