I just submitted a draft of my article about Google text ads for the September issue of RAPS Regulatory Focus. I was surprised at how much has changed since my initial piece on this topic about two years ago.
If RAPS agrees, I'll make a copy available on my Scribd.com page (which is always accessible via the link in the right rail of the blog).
I'll also be drawing upon much of that material for a presentation I'm delivering on September 12 at the CBI Social Media Summit in Philadelphia. I hope you'll join us.
Eliminating the Disparity in 2253 Filing Requirements
One of the difficulties with the guidance on Fulfilling Regulatory Requirements for Postmarketing Submissions of Interactive Promotional Media for Prescription Human and Animal Drugs and Biologics (or the 2253 guidance as I call it*) on social media is that it establishes a distinction in filing requirements between forums that are publicly accessible and those that are not.
Sponsors of prescription products who wish to engage in social media are granted two pathways via this guidance for meeting their 2253 filing requirements, which is the obligation to submit all promotional materials at the time of first use or prior to initial dissemination.
For forums that are publicly accessible, sponsors can submit the initial communication on a third-party site, along with any home or profile page, and the URL where the real-time communications will occur. Then, every month, firms should submit the list of all URLs where it is engaging in real-time communications.
However, forums that are not publicly accessible have a different requirement. Instead of merely submitting a list of URLs each month, FDA requests that sponsors who choose to use such forums submit screenshots of the interactions that occur.
It has been pointed out by others (including yours truly) that this distinction is difficult to understand or draw in practice. For example, many platforms have a minimal requirement that to view any discussions in the platform, you must be a member of (and logged in to your account on) the platform. Would such a requirement be seen by the FDA as making the site not publicly accessible?
Moreover, for third-party sites, this requirement might change without notice. Were that to happen, a sponsor could go from being in compliance to being out of compliance without having themselves made any changes, and that seems odd. It certainly seems that what a sponsor is operating compliantly should not depend on the policies of a third-party site.
Another difficulty is that the disparity in filing requirement is huge and would put a barrier in front of participating in non-restricted forums. Preparing all of the screenshots on a monthly basis could be a significant undertaking depending on the volume of the communications in the forum. While that might seem desirable at first blush, there are good reasons to want to operate such closed forum. Such forums enable, for example, full verification of credentials prior to admission. That means that information can be more freely shared without concern that people who should not have access to the information might receive it. For example, if I were the sponsor of a pain medication, I might want to discuss methods that some patients are using to circumvent abuse prevention mechanisms, but obviously I would not want that information to be available in a publicly accessible forum.
The essence behind this requirement seems to be the desire of FDA to monitor the real-time communications and to rely on the possibility of FDA monitoring of communications to ensure that all communications are appropriate.**
One way of addressing these concerns and eliminating the disparity in filing requirements is to require that when sponsors make use of third-party platforms with restricted access that they set up credentials for FDA to access the forum.
It would be easy for FDA to create an email address and name (e.g., OPDP Social Media & OPDPSocMed@FDA.gov) that could be created as a user for any restricted access platforms. To further simplify things, FDA could establish and publish a list of its credentials on the most prominent forums (e.g., YouTube, Facebook, LinkedIn, Twitter) for monitoring purposes. In that way, sponsors could simply create a user account with FDA's credentials when they set up their restricted-access forums. The sponsor could then include as part of their initial 2253 filing requirement a password (if required) for FDA to access the forum.
From that point forward, sponsors would then be on equal footing with the requirement simply to file monthly reports indicating their continued involvement in the platform but not being required to submit screenshots of all of the interactions.
This method would enable FDA to monitor the conversations in real-time without creating a disparity in filing or a barrier to establishing and maintaining restricted-access forums. It also would ensure that changes made by third-parties could not cause a sponsor to find themselves out of compliance with FDA requirements.
* It is worth noting that the guidance also affects filing of FDA Form 2301 for veterinary medicines. Nothing in this post is affected by this nuance.
** Quoting from the guidance, "if a site has restricted access and, as such, FDA may not have access to the site" (page 7, emphasis added)
Sponsors of prescription products who wish to engage in social media are granted two pathways via this guidance for meeting their 2253 filing requirements, which is the obligation to submit all promotional materials at the time of first use or prior to initial dissemination.
For forums that are publicly accessible, sponsors can submit the initial communication on a third-party site, along with any home or profile page, and the URL where the real-time communications will occur. Then, every month, firms should submit the list of all URLs where it is engaging in real-time communications.
However, forums that are not publicly accessible have a different requirement. Instead of merely submitting a list of URLs each month, FDA requests that sponsors who choose to use such forums submit screenshots of the interactions that occur.
It has been pointed out by others (including yours truly) that this distinction is difficult to understand or draw in practice. For example, many platforms have a minimal requirement that to view any discussions in the platform, you must be a member of (and logged in to your account on) the platform. Would such a requirement be seen by the FDA as making the site not publicly accessible?
Moreover, for third-party sites, this requirement might change without notice. Were that to happen, a sponsor could go from being in compliance to being out of compliance without having themselves made any changes, and that seems odd. It certainly seems that what a sponsor is operating compliantly should not depend on the policies of a third-party site.
Another difficulty is that the disparity in filing requirement is huge and would put a barrier in front of participating in non-restricted forums. Preparing all of the screenshots on a monthly basis could be a significant undertaking depending on the volume of the communications in the forum. While that might seem desirable at first blush, there are good reasons to want to operate such closed forum. Such forums enable, for example, full verification of credentials prior to admission. That means that information can be more freely shared without concern that people who should not have access to the information might receive it. For example, if I were the sponsor of a pain medication, I might want to discuss methods that some patients are using to circumvent abuse prevention mechanisms, but obviously I would not want that information to be available in a publicly accessible forum.
The essence behind this requirement seems to be the desire of FDA to monitor the real-time communications and to rely on the possibility of FDA monitoring of communications to ensure that all communications are appropriate.**
One way of addressing these concerns and eliminating the disparity in filing requirements is to require that when sponsors make use of third-party platforms with restricted access that they set up credentials for FDA to access the forum.
It would be easy for FDA to create an email address and name (e.g., OPDP Social Media & OPDPSocMed@FDA.gov) that could be created as a user for any restricted access platforms. To further simplify things, FDA could establish and publish a list of its credentials on the most prominent forums (e.g., YouTube, Facebook, LinkedIn, Twitter) for monitoring purposes. In that way, sponsors could simply create a user account with FDA's credentials when they set up their restricted-access forums. The sponsor could then include as part of their initial 2253 filing requirement a password (if required) for FDA to access the forum.
From that point forward, sponsors would then be on equal footing with the requirement simply to file monthly reports indicating their continued involvement in the platform but not being required to submit screenshots of all of the interactions.
This method would enable FDA to monitor the conversations in real-time without creating a disparity in filing or a barrier to establishing and maintaining restricted-access forums. It also would ensure that changes made by third-parties could not cause a sponsor to find themselves out of compliance with FDA requirements.
* It is worth noting that the guidance also affects filing of FDA Form 2301 for veterinary medicines. Nothing in this post is affected by this nuance.
** Quoting from the guidance, "if a site has restricted access and, as such, FDA may not have access to the site" (page 7, emphasis added)
Prescription Products Don't Exist, But I Talk About Them Anyway
I use the phrase "prescription products" quite frequently. Technically, I'm misusing the phrase, but I prefer to think of it as a shorthand term of art. By "prescription products," I mean all of those products whose promotional labeling and advertising is subject to FDA regulation. That includes prescription drugs, biologics, vaccines, and restricted medical devices.
Of course, vaccines are not usually limited by prescription, and it doesn't make sense to speak about a prescription requirement for many restricted medical devices, but I find the smaller two-word phrase less cumbersome than the rather bulky "all of those products whose promotional labeling and advertising is subject to FDA regulation."
I prefer the slight fiction of creating my own term to the more inaccurate phrase of FDA-regulated products because that broader phrase includes, of course, over-the-counter drugs, homeopathic substances, nutritional supplements, tobacco, and even food and cosmetics; however, these other products are not subject to FDA regulation of their promotional efforts, except in so far as FDA requires that their promotional efforts cannot imply that the items are in fact prescription products.
Of course, vaccines are not usually limited by prescription, and it doesn't make sense to speak about a prescription requirement for many restricted medical devices, but I find the smaller two-word phrase less cumbersome than the rather bulky "all of those products whose promotional labeling and advertising is subject to FDA regulation."
I prefer the slight fiction of creating my own term to the more inaccurate phrase of FDA-regulated products because that broader phrase includes, of course, over-the-counter drugs, homeopathic substances, nutritional supplements, tobacco, and even food and cosmetics; however, these other products are not subject to FDA regulation of their promotional efforts, except in so far as FDA requires that their promotional efforts cannot imply that the items are in fact prescription products.
PhillyCooke.com Is Now Live
As many of you know, I have recently launched an independent consulting practice, focused on helping companies figure out how to use new and emerging digital channels to provide information about FDA-regulated products to the people who need that information, whether that's patients, caregivers, or doctors and other healthcare professionals.
I'm in the process of forming an official S-Corporation (paperwork filed with and apparently accepted by the IRS and Commonwealth of Pennsylvania) and this blog has been undergoing some design changes as I've adapted it to become simply one page on the slightly larger website that is dedicated to the consulting practice.
You might have noticed the change in the page header, new navigation elements, background image, etc.
Aside from the cosmetic changes, the intention is to provide a single location where you can find information about the topic of promoting FDA-regulated products and the services I provide as a consultant. So, there's a new page dedicated to describing the services PhillyCooke Consulting offers.
If you find yourself in need of any of the services, please reach out to me. You can fill out the contact form on the right, and you'll hear from me as soon as I am able to do so.
And if you're not sure whether your specific request falls within the scope of services I provide, then please ask. I don't do everything, but I am a committed provider of referrals and would happily connect you with someone else in my Rolodex.
In addition, this blog will continue, and I hope you will continue to read it. Because although there's a change on my business card, I remain dedicated to the endlessly fascinating topic of FDA regulation of advertising and promotion of prescription products.
I'm in the process of forming an official S-Corporation (paperwork filed with and apparently accepted by the IRS and Commonwealth of Pennsylvania) and this blog has been undergoing some design changes as I've adapted it to become simply one page on the slightly larger website that is dedicated to the consulting practice.
You might have noticed the change in the page header, new navigation elements, background image, etc.
Aside from the cosmetic changes, the intention is to provide a single location where you can find information about the topic of promoting FDA-regulated products and the services I provide as a consultant. So, there's a new page dedicated to describing the services PhillyCooke Consulting offers.
If you find yourself in need of any of the services, please reach out to me. You can fill out the contact form on the right, and you'll hear from me as soon as I am able to do so.
And if you're not sure whether your specific request falls within the scope of services I provide, then please ask. I don't do everything, but I am a committed provider of referrals and would happily connect you with someone else in my Rolodex.
In addition, this blog will continue, and I hope you will continue to read it. Because although there's a change on my business card, I remain dedicated to the endlessly fascinating topic of FDA regulation of advertising and promotion of prescription products.
YouTube Channel Launched
I'm always looking to expand in my use of social media and to provide more resources for others interested in using social media for promoting prescription products, so I just launched PhillyCooke's YouTube Channel.
Here is the link, which will also be featured in the widget on the right with the other online locations where you can find resources from me.
Right now, there is only one video, a short clip from the movie The Social Network, that I really liked because as much as I love social media, I have sometimes found it a frustrating experience, as I've talked about before, trying to figure out how all of this stuff works.
Here is the link, which will also be featured in the widget on the right with the other online locations where you can find resources from me.
Right now, there is only one video, a short clip from the movie The Social Network, that I really liked because as much as I love social media, I have sometimes found it a frustrating experience, as I've talked about before, trying to figure out how all of this stuff works.
Tweet Embedding Correction
My previous post on Twitter's new Tweet embedding feature contained an error that was pointed out to me by a correspondent.
I claimed that one of the drawbacks to the way Twitter was implementing Tweet embedding is that for the feature to function, you must include the full URL of the Tweet you want to embed in the new Tweet. As I wrote:
In fact, Twitter automatically shortens the URL, so that it is not the full character count of the URL that counts against the 140-character limit for each Tweet.
However, the character count for the URL is still not as short as a typical URL shortener.
Here were the results of playing with the functionality:
Again, I used the same Tweet for the embedding:
That Tweet is available at: https://twitter.com/PhillyCooke/status/496670428187090944
The full URL is 57-characters long.
However, I can add it to a Tweet that only appears to have 24 characters remaining (i.e., the rest of the Tweet is actually 116-characters long).
Note that I'm able to comfortably insert the 57-character URL into the Tweet, and the "Tweet" button is still functioning, with an available character count of 0.
Of course, the 23 characters required by Twitter's URL shortening of its own URL is still greater than the 11 characters that I was able to get using Ow.ly, but rather than being a saving of 46 characters, as I claimed in the original post, it is a mere 12 characters that are saved.
Even so, 12 characters does constitute nearly 10% of a Tweet's 140 total characters. Such a savings is not to be ignored lightly.
Thanks to the correspondent who told me about the error, and let me know via email and/or comments if you have any other ideas for saving characters or learning more about how Twitter's embedding functionality works.
I claimed that one of the drawbacks to the way Twitter was implementing Tweet embedding is that for the feature to function, you must include the full URL of the Tweet you want to embed in the new Tweet. As I wrote:
To get the embedding to function as demonstrated above, you must include the full URL of the original Tweet in the new Tweet....the full URL is extremely long. In my example it takes up 57 characters of the 140 allowed...That was incorrect.
In fact, Twitter automatically shortens the URL, so that it is not the full character count of the URL that counts against the 140-character limit for each Tweet.
However, the character count for the URL is still not as short as a typical URL shortener.
Here were the results of playing with the functionality:
Again, I used the same Tweet for the embedding:
 |
| Original Tweet |
That Tweet is available at: https://twitter.com/PhillyCooke/status/496670428187090944
The full URL is 57-characters long.
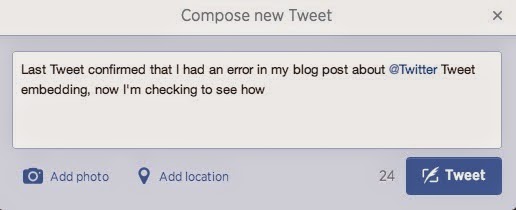
However, I can add it to a Tweet that only appears to have 24 characters remaining (i.e., the rest of the Tweet is actually 116-characters long).
 |
| New Tweet Prior to Adding the URL |
Note that I'm able to comfortably insert the 57-character URL into the Tweet, and the "Tweet" button is still functioning, with an available character count of 0.
 |
| Tweet with Full URL |
Also note that the Tweet URL took up 23 characters because I had to include a space between the last word "how" and the URL. Otherwise, Twitter doesn't acknowledge the URL as being an embedded Tweet and simply prevents me from sending out the Tweet.
 |
| Tweet without Space Showing It Is Too Long |
In this case, you can see that the URL isn't recognized as such by Twitter. Consequently, the character count shows up as -33, and the "Tweet" button is grayed out showing that it is inactive.
All of the above screen shots were taken from Twitter.com on a Macbook Air using Chrome.
I also checked how this functionality worked on HootSuite, which I have previously mentioned is my preferred means of accessing Twitter on my laptop. And the same shortening behavior happened.
 |
| Tweet on Hootsuite Prior to Sending |
Of course, the 23 characters required by Twitter's URL shortening of its own URL is still greater than the 11 characters that I was able to get using Ow.ly, but rather than being a saving of 46 characters, as I claimed in the original post, it is a mere 12 characters that are saved.
Even so, 12 characters does constitute nearly 10% of a Tweet's 140 total characters. Such a savings is not to be ignored lightly.
Thanks to the correspondent who told me about the error, and let me know via email and/or comments if you have any other ideas for saving characters or learning more about how Twitter's embedding functionality works.
Social Media Webinar
I'm presenting a webinar for LSTI on Wednesday, October 8.
In the webinar, I'll be taking a step back from the individual guidances we've received and try to give marketers and others interested in the use of social media for promoting prescription products the 50,000-foot view.
By doing so, I hope to provide a roadmap for people who are struggling to determine what they can expect from FDA and how to move forward on implementing social media initiatives.
Here's the link to the full description and registration information: http://www.lifesciencetraininginstitute.com/doc/fda-guidance-on-social-media-questions-answered-and-unanswered-0001
Hope you'll join!
In the webinar, I'll be taking a step back from the individual guidances we've received and try to give marketers and others interested in the use of social media for promoting prescription products the 50,000-foot view.
By doing so, I hope to provide a roadmap for people who are struggling to determine what they can expect from FDA and how to move forward on implementing social media initiatives.
Here's the link to the full description and registration information: http://www.lifesciencetraininginstitute.com/doc/fda-guidance-on-social-media-questions-answered-and-unanswered-0001
Hope you'll join!
PHL RAPS Meeting September 9
Update: Registration is now live: http://www.raps.org/EventDetail.aspx?id=19972#horizontalTab3
Our next local RAPS meeting will be held Tuesday, September 9, again at the Primavera Pizza Kitchen in Ardmore. The event will lead up to the RAPS national conference in Austin, TX, Sept. 27 – Oct. 1. Here’s the online registration for the national conference: http://connect.raps.org/2014raps/home/?ssopc=1
Linda Bowen of sanofi will be joining us to present conference overview, and Alec Gaffney, who reports for RAPS Regulatory Focus, will be providing an exclusive preview of the panel discussion he’s participating in at RAPS national about using technology for regulatory intelligence.
Some of you might know Alec for his recently published lists of 460 to Follow and RSS Feeds for Regulatory Pros. I'm proud to say that I'm included on both lists and am really looking forward to hearing from Alec himself on how he manages to stay on top of so much information.
Our next local RAPS meeting will be held Tuesday, September 9, again at the Primavera Pizza Kitchen in Ardmore. The event will lead up to the RAPS national conference in Austin, TX, Sept. 27 – Oct. 1. Here’s the online registration for the national conference: http://connect.raps.org/2014raps/home/?ssopc=1
Linda Bowen of sanofi will be joining us to present conference overview, and Alec Gaffney, who reports for RAPS Regulatory Focus, will be providing an exclusive preview of the panel discussion he’s participating in at RAPS national about using technology for regulatory intelligence.
Some of you might know Alec for his recently published lists of 460 to Follow and RSS Feeds for Regulatory Pros. I'm proud to say that I'm included on both lists and am really looking forward to hearing from Alec himself on how he manages to stay on top of so much information.
Embedding Tweets?
Update: This post contains an error about the character counts of the URLs. That error was corrected via a more recent post, which can be accessed here: http://regulatoryrx.blogspot.com/2014/08/tweet-embedding-correction.html
Last week, Media Bistro had a story about Twitter enabling the embedding of Tweets, and several people asked me whether this would be useful for pharma to make use of social media and provide more information.
Here is a sample progression that I sent out much to my followers' annoyance (sorry about that!).
Original Tweet:
You won't always see exactly that same thing.
For example, here's what the Tweet looked like in my Hootsuite tab (again from Chrome on Macbook Air):
Again, the full original Tweet is displayed with full attribution (Twitter handle, user name, and Twitter avatar).
Tapping the new Tweet (with the embedded Tweet inside it) takes you to a full screen display of the message.
By contrast, tapping on the embedded Tweet opens only that Tweet in full screen.
From the perspective of achieving compliance for using Twitter for marketing prescription products, I don't see how this will help much. While it is true that some people would be able to receive more information via a Tweet that contains an embedded Tweet (e.g., you could embed a Tweet dedicated solely to providing risk information), the variety in appearance is concerning. I wouldn't feel comfortable relying on the fact that people would access my Tweet via Twitter.com or via the official Twitter app, instead of using a third-party platform, such as Hootsuite.
Indeed, when I'm on my laptop, I almost exclusively use Hootsuite to access Twitter, rather than using Twitter.com; so unless there were a means to restrict access to the message to people using platforms that accommodate Tweet embedding, I'd be averse to relying on this mechanism to provide mandatory information in a Tweet (such as risk information).
I also am not sure whether the FDA would regard information presented via an embedded Tweet as having comparable prominence to information presented in the primary Tweet. That's important for the presentation of risk information to meet the fair balance requirement.
There is another problem with this method of providing information.
To get the embedding to function as demonstrated above, you must include the full URL of the original Tweet in the new Tweet. Here's what that looks like:
As you can see, the full URL is extremely long. In my example it takes up 57 characters of the 140 allowed, and if you use a URL shortener, then the functionality fails. Here's the view on Hootsuite:
Of course, embedded Tweets don't show on Hootsuite, so I also checked on Twitter.com. Here's that view:
And I checked the mobile view in the official Twitter app.
In both cases, only the shortened URL was displayed. That's a real shame as the savings of 46 characters (11 for shortened URL vs. 57 for the full URL) is huge in the realm of Tweets.
When I looked on mobile, I noticed another interesting feature. Namely, that while viewing your own Tweets from the Me portion of the app, the embedded Tweet doesn't appear, even when you provide the full URL. It actually looks the same as the Hootsuite view, presenting just the beginning of the full URL.
So, while embedding Tweets could certainly play a role in providing Twitter users with a way to include much more valuable information in this important platform, I'm afraid that at the moment it won't significantly expand the ability to use Twitter compliantly for manufacturers of prescription products.
h/t to Alec Gaffney & Polaris Consulting for this post.
Last week, Media Bistro had a story about Twitter enabling the embedding of Tweets, and several people asked me whether this would be useful for pharma to make use of social media and provide more information.
Here is a sample progression that I sent out much to my followers' annoyance (sorry about that!).
Original Tweet:
 |
| Original Tweet on Twitter.com |
Here's the Tweet with the embedded portion as it appears on Twitter.com accessed from my Macbook Air using Chrome:
 |
| Embedded Tweet on Twitter.com |
You won't always see exactly that same thing.
For example, here's what the Tweet looked like in my Hootsuite tab (again from Chrome on Macbook Air):
 |
| Embedded Tweet on Hootsuite |
Note the important differences that the embedded Tweet appears fully as a picture-like object with the new Tweet on Twitter.com, but when using Hootsuite, you only see the URL for the original Tweet, and even that URL is cut off, so any information contained in the original Tweet only comes through on some platforms.
The mobile experience is also varied.
I use the official Twitter app on my iPhone.
Here are the different views there. The first is what I see in my Twitter feed from the Home screen, showing my full Twitter stream:
 |
| Mobile View in Stream |
Tapping the new Tweet (with the embedded Tweet inside it) takes you to a full screen display of the message.
 |
| Mobile View 2: Full Screen Display of Tweet |
By contrast, tapping on the embedded Tweet opens only that Tweet in full screen.
 |
| Original Tweet on Mobile from Tapping on Embedded Portion |
From the perspective of achieving compliance for using Twitter for marketing prescription products, I don't see how this will help much. While it is true that some people would be able to receive more information via a Tweet that contains an embedded Tweet (e.g., you could embed a Tweet dedicated solely to providing risk information), the variety in appearance is concerning. I wouldn't feel comfortable relying on the fact that people would access my Tweet via Twitter.com or via the official Twitter app, instead of using a third-party platform, such as Hootsuite.
Indeed, when I'm on my laptop, I almost exclusively use Hootsuite to access Twitter, rather than using Twitter.com; so unless there were a means to restrict access to the message to people using platforms that accommodate Tweet embedding, I'd be averse to relying on this mechanism to provide mandatory information in a Tweet (such as risk information).
I also am not sure whether the FDA would regard information presented via an embedded Tweet as having comparable prominence to information presented in the primary Tweet. That's important for the presentation of risk information to meet the fair balance requirement.
There is another problem with this method of providing information.
To get the embedding to function as demonstrated above, you must include the full URL of the original Tweet in the new Tweet. Here's what that looks like:
 |
| Tweet with Full URL Displayed |
As you can see, the full URL is extremely long. In my example it takes up 57 characters of the 140 allowed, and if you use a URL shortener, then the functionality fails. Here's the view on Hootsuite:
 |
| Embedded Tweet Using URL Shortener on Hootsuite |
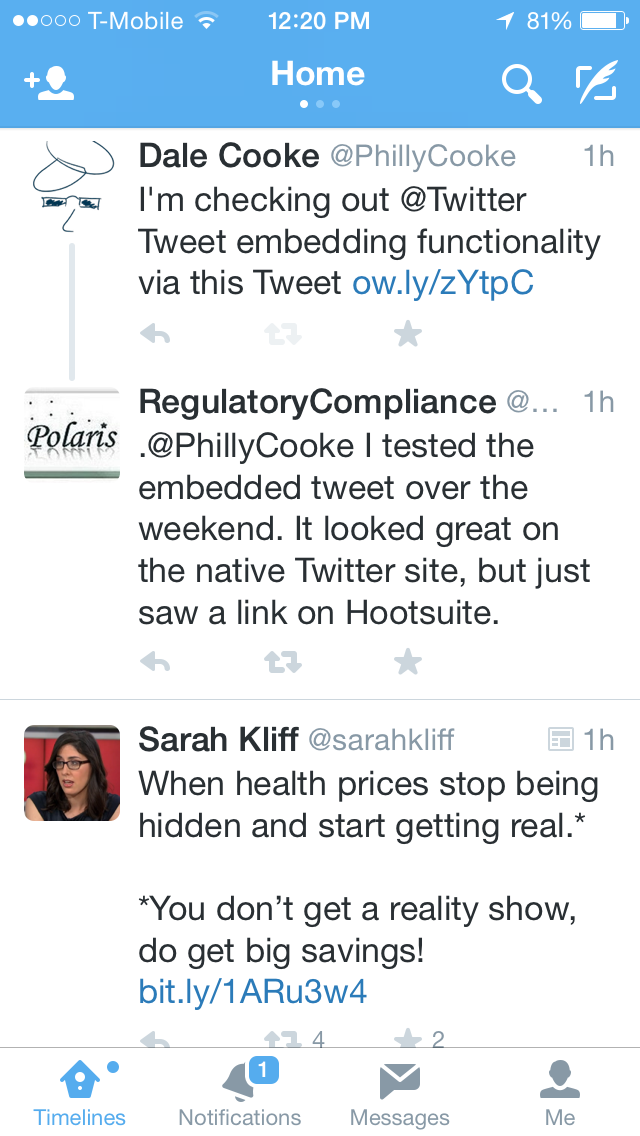
Of course, embedded Tweets don't show on Hootsuite, so I also checked on Twitter.com. Here's that view:
 |
| URL Shortener Usage on Twitter.com |
 |
| URL Shortener Usage on Mobile in Stream |
In both cases, only the shortened URL was displayed. That's a real shame as the savings of 46 characters (11 for shortened URL vs. 57 for the full URL) is huge in the realm of Tweets.
When I looked on mobile, I noticed another interesting feature. Namely, that while viewing your own Tweets from the Me portion of the app, the embedded Tweet doesn't appear, even when you provide the full URL. It actually looks the same as the Hootsuite view, presenting just the beginning of the full URL.
 |
| Multiple Embedded Tweets on Mobile Showing Shortener Usage |
h/t to Alec Gaffney & Polaris Consulting for this post.
Save Money While Learning about Google Text Ads
As I mentioned previously, I'll be speaking at CBI's one-day event on social media. CBI has confirmed that I can offer a $300 discount to anyone who contacts me via this blog.
Just fill out the contact form on the right, and I'll send you the promotional code by email.
Just fill out the contact form on the right, and I'll send you the promotional code by email.
460 to Follow
Alec Gaffney of RAPS has compiled an extremely valuable list of Twitter accounts for people in regulatory affairs to follow. The list includes governmental accounts, members of the press, industry accounts, and consultants such as yours truly.
I'm very pleased to be included in the list, and if you're looking to start using Twitter for regulatory intelligence, I strongly recommend that you start by combing this list for the accounts that most fit your interests.
Here's the link to the full list: http://www.raps.org/regulatory-focus/twitter/
I'm very pleased to be included in the list, and if you're looking to start using Twitter for regulatory intelligence, I strongly recommend that you start by combing this list for the accounts that most fit your interests.
Here's the link to the full list: http://www.raps.org/regulatory-focus/twitter/
Subscribe to:
Posts (Atom)